Immunogenicity of HPV Vaccines
International and domestic studies have demonstrated that after completing the full vaccination schedule with bivalent, quadrivalent, and nonavalent (nine-valent) HPV vaccines, high seroconversion rates for vaccine-type HPV antibodies and high levels of antibody GMCs can be observed [1]. Clinical studies in China have shown that females aged 9-17 years exhibit a robust immune response after receiving bivalent and quadrivalent HPV vaccines, with antibody titers being 1.42-3.00 times higher than those in females aged 18-26 years. The antibody titers in females aged 18-25 are similar to those in females aged 26-45 [2].
Efficacy and Safety of HPV Vaccines
HPV Bivalent Vaccine (Cervarix)
A multi-country, double-blind, randomized-controlled trial (PATRICIA) sponsored by GlaxoSmithKline included 18,644 healthy female subjects aged 15-25 years – who reported no more than six lifetime sexual partners before study enrolment, agreed to adequate contraception over the vaccination period and had an intact cervix were eligible for inclusion. The total vaccinated cohort (TVC) included all randomized participants who received at least one vaccine dose (vaccine, n=9,319; control, n= 9,325) at months 0, 1, and/or 6. The TVC-naive (vaccine, n = 5,822; control, n = 5,819) had no evidence of high-risk HPV infection at baseline, approximating adolescent girls targeted by most HPV vaccination programs [3].
In the TVC, vaccine efficacy (VE) against cervical intraepithelial neoplasia (CIN) grade 1 or greater (CIN1+), CIN2+, and CIN3+ associated with HPV-16/18 was 55.5% (96.1% CI: 43.2-65.3), 52.8% (96.1% CI: 37.5-64.7), and 33.6% (96.1% CI: −1.1-56.9). In the TVC-naive, VE against CIN1+, CIN2+, and CIN3+ associated with HPV-16/18 was 96.5% (96.1% CI: 89.0-99.4), 98.4% (96.1% CI: 90.4-100), and 100% (96.1% CI: 64.7-100). VE against 12-month persistent infection with HPV-16/18 was 89.9% (96.1% CI: 84.0-94.0), and that against HPV-31/33/45/51 was 49.0% (96.1% CI: 34.7-60.3) [3]. Vaccine efficacy against all Adenocarcinoma in situ (AIS) was 100% (95% CI: 31.0-100) and 76·9% (95% CI: 16.0-95.8) in the TVC-naive and TVC, respectively [4].
The Phase III, double-blind, randomized controlled VIVIANE study enrolled healthy females aged 25 years and above from twelve countries. After 7 years of follow-up, vaccine efficacy against 6-month persistent infection or CIN1+ associated with HPV 16/18 was significant in all age groups combined (90·5%, 96·2% CI:78·6-96·5). Cross-protective efficacy against 6-month persistent infection with HPV 31 and HPV 45 was also significant. Serious adverse events related to vaccination occurred in five (0·2%) of 2877 women in the vaccine group and eight (0·3%) of 2870 women in the control group [5].
Results from a Phase II/III randomized, controlled trial among healthy females aged 18-25 years in China indicated that up to 72 months after the first vaccination, among the initially HPV-16/18 seronegative/DNA-negative women, vaccine efficacy against HPV-16/18-associated CIN grade 2 or worse was 87.3% (95% CI: 5.5-99.7) in the according-to-protocol efficacy cohort (ATP-E), 88.7% (95% CI: 18.5-99.7) in the total vaccinated cohort for efficacy (TVC-E), and 100% (95% CI: 17.9-100) in the TVC-naïve cohort. Serious adverse events were infrequent based on the observational results of approximately 57 and 72 months [6,7].
HPV Bivalent Vaccine (Cecolin)
An interim analysis of a multicenter, randomized, double-blind, placebo-controlled study involving 7372 Chinese females aged 18-45 years revealed that after 42 months of follow-up, those who received three doses of the domestic bivalent HPV vaccine (Escherichia coli) exhibited 100% efficacy in protecting against HPV16/18-related CIN 2/ 3, AIS, or cervical cancer. The vaccine demonstrated 97.8% efficacy in preventing persistent genital HPV infection. No vaccine-related severe adverse events were observed, and both types of neutralizing antibodies remained sustained for at least 42 months [8].
The follow-up study conducted 66 months after receiving three doses of the E coli-produced bivalent vaccine showed the vaccine was well tolerated and highly efficacious. In the per-protocol population, the vaccine reveals a 100% efficacy (95% CI: 67.2-100.0) in preventing high-grade genital lesions associated with HPV 16/18. Additionally, the vaccine showed a 97.3% efficacy (95% CI: 89.9-99.7) in preventing persistent infections caused by HPV16/18. The incidence of serious adverse events was comparable between the vaccine group, which included 267 out of 3691 participants (7.2%), and the control group, with 290 out of 3681 participants (7.9%) experiencing such events. These serious adverse events were deemed to be not related to the bivalent HPV vaccine [9].
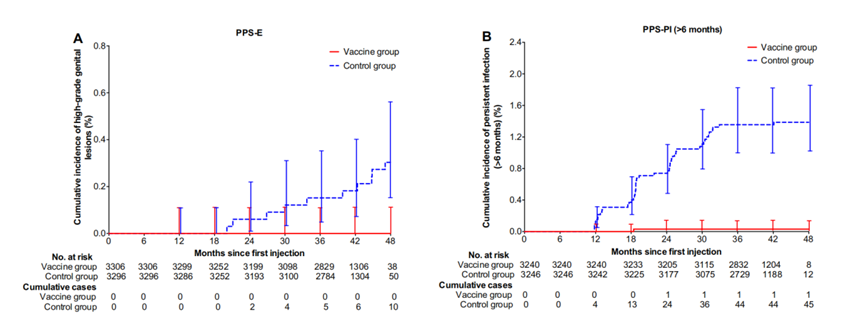
Figure 1. Duration of HPV-16/18-Related High-Grade Genital Lesions or Persistent Infections in Susceptible Populations in the Vaccine and Control Groups (Image Source: Efficacy, Safety, and Immunogenicity of an Escherichia coli-Produced Bivalent Human Papillomavirus Vaccine: An Interim Analysis of a Randomized Clinical Trial)
Quadrivalent HPV Vaccine (Gardasil 4)
A double-blind, placebo-controlled trial involving 12,167 women aged 15 to 26 across 13 countries (non-pregnant, with normal Pap smear results and no more than four sexual partners in the past) was conducted to evaluate the efficacy of the quadrivalent HPV vaccine in preventing high-grade cervical lesions associated with HPV16/18. The primary composite endpoint was CIN2/3, AIS, or cervical cancer related to HPV16/18. The subjects received an average of three years of follow-up after the first dose of the vaccine or placebo, with the vaccine demonstrating efficacy of 98% (95.89% CI: 86-100) in preventing the primary composite endpoint [10].
A meta-analysis of four clinical studies evaluated the impact of prophylactic vaccination with the quadrivalent HPV vaccine on CIN2/3 and AIS. The four studies included 20,583 women aged 16-26, with an average follow-up of three years after the first dose. The primary endpoint was the combined incidence of CIN2/3, carcinoma in situ, or cervical cancer related to HPV16/18. In women who were negative for HPV16/18 infection during the vaccination period, the vaccine’s efficacy in preventing the primary endpoint was 99% (95% CI: 93-100); an intention-to-treat (ITT) analysis for all women (including those infected with HPV16/18) showed an efficacy of 44%. The ITT analysis also indicated a reduction in the overall incidence of CIN2/3 or carcinoma in situ caused by all HPV types by 18% (95% CI: 7-29) [11]. A study conducted in the Nordic countries (Denmark, Iceland, Norway, and Sweden) evaluated the long-term effects of the quadrivalent HPV vaccine. It supported that the vaccine’s protection can be sustained for at least 10 years [12].
For women who have already been infected with one to three HPV viruses, the quadrivalent HPV vaccine can also prevent tumors caused by other HPV viruses [13]. In addition, based on an analysis of three randomized clinical trials, prophylactic vaccination with the quadrivalent HPV vaccine was also effective in preventing high-grade vulvar and vaginal lesions caused by HPV16/18 infections [14].
A clinical study in China involving females aged 20-45, with a follow-up of 78 months, demonstrated that the quadrivalent HPV vaccine provided 100% protection against CIN2/3, AIS, and cervical cancer related to HPV 16/18. The vaccine also reduced cytological abnormalities associated with HPV 6, 11, 16, and 18, with an efficacy rate of 94.0% [15]. Furthermore, a randomized, double-blind, placebo-controlled trial involving Chinese women aged 20 to 45, conducted over 90 months, revealed that the incidence of systemic adverse reactions within 15 days of vaccination was similar between the vaccine and placebo groups (46.8% versus 45.1%). However, the rate of injection site adverse reactions was notably higher in the vaccine group (37.6% versus 27.8%). Serious adverse events were reported by 38 participants in the vaccine group and 43 in the placebo group, and these events were determined to be unrelated to the HPV vaccination [16].
Nine-valent HPV Vaccine(Gardasil 9)
Several clinical studies targeting various age groups and genders have validated the immunogenicity and clinical efficacy of the nine-valent HPV vaccine.
Age 9-15:
A clinical Phase III study conducted abroad included 1272 males and females aged 9-15 with no sexual activity and no sexual activity plans within six months. The study found that recipients of three doses of the nonavalent HPV vaccine reached peak neutralizing antibody titers at 7 months, which remained above 90% (Luminex immunoassay) even after 90 months. No high-grade intraepithelial lesions or genital warts related to HPV 6/11/16/18/31/33/45/52/58 were observed during the 8.2-year follow-up [17]. A subsequent study demonstrated that 11 years (median of 10 years) after the administration of three doses of the nonavalent HPV vaccine, the seropositivity rates for each HPV type among the subjects remained above 81%, as measured by the Luminex immunoassay. No cases of high-grade intraepithelial neoplasia or genital warts associated with HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 were observed among the subjects [18].
Age 16-26:
A randomized, double-blind, efficacy, immunogenicity, and safety clinical study conducted across 105 research centers in 18 countries enrolled 14,215 women aged 16-26 (with no history of cervical cytological abnormalities and no more than four past sexual partners). Participants were randomly assigned in a 1:1 ratio to receive three doses of either the quadrivalent or nonavalent vaccine. The primary outcomes were the incidence rates of high-grade cervical, vulvar, and vaginal diseases. The study found that the nonavalent vaccine had similar efficacy to the four-valent vaccine in preventing the shared 4 HPV types. Additionally, the nonavalent vaccine shows 96.7%(95%CI: 80.9-99.8)efficacy in preventing high-grade cervical intraepithelial neoplasia, vaginal intraepithelial neoplasia, vulvar and vaginal cancers related to the HPV types 31, 33, 45, 52, and 58, albeit with a higher proportion of adverse reactions at the injection site [19]. Data from two international studies, encompassing Asian women from India, Hong Kong (China), South Korea, Japan, Taiwan (China), and Thailand, indicate that the nonavalent HPV vaccine has an efficacy rate ranging from 90.4% to 100% in preventing persistent infections associated with HPV types 31, 33, 45, 52, and 58 [20]. The vaccine’s efficacy was maintained at a certain level over the 6-year follow-up period, with four severe adverse events observed in participants who received the nonavalent vaccine, similar to the quadrivalent vaccine group [21].
Age 27-45:
A recent Phase III randomized controlled trial involving 1,212 healthy females from multiple countries confirmed the non-inferiority of neutralizing antibody titers against HPV 16, 18, 31, 33, 45, 52, and 58 in females aged 27-45 compared to those aged 16-26 after receiving three doses of the nonavalent HPV vaccine. For all HPV types, the seroconversion rate exceeded 99% in the seventh month (seven months after the first dose at day 0). Adverse events at the injection site and vaccine-related systemic adverse events occurred in 85.2% and 24.1% of females aged 27-45, respectively, with no reports of severe vaccine-related adverse events [22].
Chinese female population:
A Phase III, non-randomized, open-label clinical trial was conducted to assess the immunogenicity and safety of the nonavalent HPV vaccine (Gardasil 9) among Chinese women aged 9-45. 1,990 participants were enrolled, divided into three age groups: 690 individuals aged 9-19, 650 aged 20-26, and 650 aged 27-45. All participants received three doses of the nonavalent HPV vaccine. The seropositivity rates of antibodies one month after the third dose were compared with the levels before the vaccination. The study results indicated that the seropositivity rates in the 9-19 and 27-45 age groups were non-inferior to those in the 20-26 age group. Adverse reactions at the injection site were reported by 43.3%, 50.5%, and 43.8% of the participants in the 9-19, 20-26, and 27-45 age groups, respectively. Additionally, systemic adverse reactions were reported by 50.9%, 57.1%, and 43.4% of the participants in the respective age groups.
Domestic nonavalent HPV vaccine (Cecolin 9, not yet approved for market)
A single-center, randomized, single-blind Phase III clinical trial was conducted in China, enrolling 553 women aged 18-26. Participants were randomly assigned in a 1:1 ratio to receive either the Cecolin-9 or Gardasil-9 vaccine and underwent a head-to-head comparison of immunogenicity. The study showed that the HPV type-specific immune response induced by Cecolin 9 was non-inferior to that induced by Gardasil 9.
Efficacy in Special Populations
Male Population
A Cochrane review revealed moderate-quality evidence suggesting that vaccination with the quadrivalent HPV vaccine in males aged 10-26 reduced the incidence of external genital lesions and anal genital warts compared to the control group [25]. A randomized controlled trial conducted in Japan found that the quadrivalent HPV vaccine demonstrated an 85.9% efficacy against HPV 6, 11, 16, and 18 infections. Although the serum antibody positivity rate declined between 7 and 36 months, most recipients maintained a certain level of seropositivity and serum antibody titers after 36 months [26]. Another community-based randomized controlled trial conducted in Finland indicated that high coverage of HPV vaccination in boys could bring public health benefits to unvaccinated females [27].
HIV-Positive Population
A systematic review examined studies on HPV vaccine administration in HIV-infected individuals and found that vaccination with the bivalent or quadrivalent HPV vaccine in HIV-infected women was safe and immunogenic [28]. A study involving HIV-infected women aged 18-25 in South Africa found that the immune response after receiving the bivalent HPV vaccine was not affected by CD4 T cell count or HIV viral load [29]. Another study involving 319 HIV-infected women aged 13-45 in the United States, Brazil, and South Africa found that after 28 weeks of vaccination with the quadrivalent HPV vaccine, the seroconversion proportions of HPV types ranged from 85-100% among individuals with CD4 T cells >200 cells/mm3 and 75-93% in those with CD4 T cells ≤200 cells/mm3 [30].
Efficacy of Different Dosing Regimens
Single Dose
In 2022, the World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) reviewed the evidence from studies on single-dose HPV vaccination conducted over the past few years. It concluded that the efficacy of a single-dose HPV vaccination schedule is comparable to that of a two- or three-dose regimen. The WHO then updated its position paper on HPV vaccines, recommending a one- or two-dose vaccination schedule for girls aged 9-14 (the highest priority group), a one- or two-dose schedule for girls and women aged 15-20, and a two-dose schedule for women over 21, with a six-month interval between doses [31].
Since 2018, the HPV Vaccine Impact Assessment Coalition, coordinated by PATH, has been collecting data from clinical trials, observational studies, and modeling analyses [32]. The accumulated evidence to date supports the immunization schedule of a single dose of HPV vaccine, which can reduce the incidence of precancerous lesions and cancer in girls and young women aged 9-14 and 15-20 as the primary target populations.
A study conducted in Costa Rica (The CVT Trial) compared the differences in high-risk HPV16/18 infection rates nearly 11.3 years after 18-25-year-old women received one, two, or three doses of the bivalent HPV vaccine (Cervarix). The study found that the vaccine efficacy against HPV16/18 was 80.2% (95% CI = 70.7%-87.0%) for women who received three doses, 83.8% (95% CI = 19.5%-99.2%) for those who received two doses, and 82.1% (95% CI = 40.2%-97.0%) for those who received only one dose. Although the antibody levels in women who received only one dose were relatively lower than in other groups, the antibody levels of women who received different doses tended to stabilize over time. The study showed a single dose of bivalent HPV vaccine may induce sufficiently durable protection for HPV16/18 [33]. Another study conducted in India also confirmed that, in women aged 10-18, a single dose of the quadrivalent HPV vaccine (Gardasil 4) still had an efficacy of 95.4% (95% CI: 85.0-99.9) in preventing persistent infection with HPV16 and 18 ten years later [34].
Recently published data from two clinical randomized controlled trials (the KEN SHE trial in Kenya and the DoRIS trial in Tanzania) further support the HPV vaccine single-dose schedule. The KEN SHE trial, a multicenter, randomized, double-blind, controlled trial, enrolled 2,275 healthy women aged 15-20, who were randomly assigned to receive bivalent (Cervarix, n=760), nonavalent (Gardasil 9, n=758), or control group vaccines. Three years after a single dose of the bivalent or nonavalent vaccine, the vaccines still had extremely high efficacy in preventing persistent HPV infection, with the nonavalent vaccine’s VE at 98.8% (95% CI: 91.3-99.8) and the bivalent vaccine at 97.5% (95% CI: 90.0-99.4) [35].
The DoRIS trial in Tanzania is an open-label, randomized, Phase III, non-inferiority trial with 1,002 healthy girls aged 9-14 who were randomly assigned to receive one, two, or three doses of the bivalent HPV vaccine (Cervarix) or one, two, or three doses of the nonavalent HPV vaccine (Gardasil 9). The study results showed that, compared to the two- and three-dose groups, the single-dose groups of both HPV vaccines met the requirements for non-inferiority in HPV-16 seropositivity rates at 24 months, even with a more stringent confidence interval. Although the seropositivity rates for HPV-18 antibodies did not meet non-inferiority, at least 98% of the girls in the single-dose groups of both vaccines had positive HPV-18 antibodies at 24 months [36].
Two Doses vs. Three Doses
A Cochrane review showed moderate to high-quality evidence that antibody responses after two-dose and three-dose HPV vaccine administration in girls aged 9-15 were similar within a 5-year follow-up period, with limited data on differences in adverse reactions [25]. A multicenter study conducted in 15 countries compared antibody responses in girls and boys aged 9-14 who received two doses of the nonavalent HPV vaccine with those of young women aged 16-26 who received three doses. The study found that four weeks after the final dose, the antibody response in girls and boys who received two doses was non-inferior to that in adolescent girls and young women who received three doses [37].
Vaccine Injection Intervals
A review of four randomized controlled trials found moderate to high-quality evidence indicating that children aged 9-14 with longer intervals (6 or 12 months) between the first two HPV vaccine doses had stronger antibody responses than those with shorter intervals (2 or 6 months) over a three-year follow-up period. There is currently no evidence from randomized controlled trials on clinical outcomes related to serious adverse events, with low-quality evidence on the occurrence of severe adverse events [25].
Safety
The HPV vaccine position paper released by the World Health Organization (WHO) in 2022 indicated that existing evidence suggests that the currently available HPV vaccines have a good safety profile [31]. The main adverse reactions manifest as mild or transient local reactions at the injection site (including erythema, pain, or swelling).
In 2018, a retrospective literature review based on 109 studies involving more than 2.5 million vaccine receivers from six countries showed that the safety of the currently available bivalent HPV vaccine (Cervarix), quadrivalent HPV vaccine (Gardasil 4), and nonavalent HPV vaccine (Gardasil 9) is acceptable, with the injection site adverse reactions of the nonavalent HPV vaccine being slightly higher than those of the quadrivalent HPV vaccine; there is no consistent evidence to suggest an increased risk of any specific adverse events of particular interest (AESI) [38]. Although data are limited, no serious adverse event outcomes were observed in pregnant women or HIV-positive children after vaccination. However, these populations’ vaccination should still be cautiously administered [39].
Content Reviewer: Kelly Hunter, Zhangyang Pan, Menglu Jiang
Page Editor: Jiaqi Zu
References:
- Chinese Society of Gynecologic Oncology, Chinese Society of Eugenics, Colposcopy and Cervical Pathology: Chinese expert consensus on the clinical application of human papillomavirus vaccine. Chinese Journal of Medical Frontiers 2021, 13(2):1-12.
- Zhu F, Li J, Hu Y, Zhang X, Yang X, Zhao H, Wang J, Yang J, Xia G, Dai Q: Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese girls and women aged 9 to 45 years: results from 2 randomized controlled trials. Human vaccines & immunotherapeutics 2014, 10(7):1795-1806.
- Apter D, Wheeler CM, Paavonen J, Castellsagué X, Garland SM, Skinner SR, Naud P, Salmerón J, Chow S-N, Kitchener HC: Efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomised, double-blind PATRICIA trial. Clinical and Vaccine Immunology 2015.
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J: Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology 2012, 13(1):89-99.
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, Salmerón J, McNeil S, Stapleton JT, Bouchard C et al: Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 2016, 16(10):1154-1168.
- Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, Zhang YJ, Pan QJ, Zhao FH, Yu JX et al: Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial. Int J Cancer 2014, 135(11):2612-2622.
- Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, Pan QJ, Zhang WH, Zhao FH, Zhang CF et al: Efficacy, immunogenicity and safety of the AS04-HPV-16/18 vaccine in Chinese women aged 18-25 years: End-of-study results from a phase II/III, randomised, controlled trial. Cancer Med 2019, 8(14):6195-6211.
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ et al: Efficacy, Safety, and Immunogenicity of an Escherichia coli-Produced Bivalent Human Papillomavirus Vaccine: An Interim Analysis of a Randomized Clinical Trial. J Natl Cancer Inst 2020, 112(2):145-153.
- Zhao, F. H., Wu, T., Hu, Y. M., Wei, L. H., Li, M. Q., Huang, W. J., … & Xia, N. S. (2022). Efficacy, safety, and immunogenicity of an Escherichia coli-produced human papillomavirus (16 and 18) L1 virus-like-particle vaccine: end-of-study analysis of a phase 3, double-blind, randomised, controlled trial. The Lancet Infectious Diseases, 22(12), 1756-1768.
- FUTURE II Study Group (2007). Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. The New England journal of medicine, 356(19), 1915–1927. https://doi.org/10.1056/NEJMoa061741
- Ault, K. A., & Future II Study Group (2007). Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet (London, England), 369(9576), 1861–1868. https://doi.org/10.1016/S0140-6736(07)60852-6
- Kjaer SK, Nygård M, Dillner J, Brooke Marshall J, Radley D, Li M, Munk C, Hansen BT, Sigurdardottir LG, Hortlund M et al: A 12-Year Follow-up on the Long-Term Effectiveness of the Quadrivalent Human Papillomavirus Vaccine in 4 Nordic Countries. Clin Infect Dis 2018, 66(3):339-345.
- FUTURE II Study Group (2007). Prophylactic efficacy of a quadrivalent human papillomavirus (HPV) vaccine in women with virological evidence of HPV infection. The Journal of infectious diseases, 196(10), 1438–1446. https://doi.org/10.1086/522864
- Joura, E. A., Leodolter, S., Hernandez-Avila, M., Wheeler, C. M., Perez, G., Koutsky, L. A., Garland, S. M., Harper, D. M., Tang, G. W., Ferris, D. G., Steben, M., Jones, R. W., Bryan, J., Taddeo, F. J., Bautista, O. M., Esser, M. T., Sings, H. L., Nelson, M., Boslego, J. W., Sattler, C., … Paavonen, J. (2007). Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet (London, England), 369(9574), 1693–1702. https://doi.org/10.1016/S0140-6736(07)60777-6
- Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, Wang S, Liao X, Shou Q, Qiu Y et al: Efficacy of quadrivalent human papillomavirus vaccine against persistent infection and genital disease in Chinese women: A randomized, placebo-controlled trial with 78-month follow-up. Vaccine 2019, 37(27):3617-3624.
- Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, Wang S, Liao X, Shou Q, Zheng M et al: Safety of a quadrivalent human papillomavirus vaccine in a Phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine 2019, 37(6):889-897.
- Olsson SE, Restrepo JA, Reina JC, Pitisuttithum P, Ulied A, Varman M, Van Damme P, Moreira ED, Jr., Ferris D, Block S et al: Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res 2020, 10:100203.
- Restrepo, J., Herrera, T., Samakoses, R., Reina, J. C., Pitisuttithum, P., Ulied, A., Bekker, L. G., Moreira, E. D., Olsson, S. E., Block, S. L., Hammes, L. S., Laginha, F., Ferenczy, A., Kurman, R., Ronnett, B. M., Stoler, M., Bautista, O., Gallagher, N. E., Salituro, G., Ye, M., … Luxembourg, A. (2023). Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety. Pediatrics, 152(4), e2022060993. https://doi.org/10.1542/peds.2022-060993
- Joura, E. A., Giuliano, A. R., Iversen, O. E., Bouchard, C., Mao, C., Mehlsen, J., … & Luxembourg, A. (2015). A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. New England Journal of Medicine, 372(8), 711-723.
- Garland SM, Pitisuttithum P, Ngan HYS, Cho CH, Lee CY, Chen CA, Yang YC, Chu TY, Twu NF, Samakoses R et al: Efficacy, Immunogenicity, and Safety of a 9-Valent Human Papillomavirus Vaccine: Subgroup Analysis of Participants From Asian Countries. The Journal of Infectious Diseases 2018, 218(1):95-108.
- Huh WK, Joura EA, Giuliano AR, Iversen O-E, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL et al: Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. The Lancet 2017, 390(10108):2143-2159.
- Joura EA, Ulied A, Vandermeulen C, Rua Figueroa M, Seppä I, Hernandez Aguado JJ, Ahonen A, Reich O, Virta M, Perino A et al: Immunogenicity and safety of a nine-valent human papillomavirus vaccine in women 27–45 years of age compared to women 16–26 years of age: An open-label phase 3 study. Vaccine 2021, 39(20):2800-2809.
- Lv, H., Wang, S., Liang, Z., Yu, W., Yan, C., Chen, Y., … & Chen, Z. (2022). Immunogenicity and safety of the 9-valent human papillomavirus vaccine in Chinese females 9–45 years of age: a phase 3 open-label study. Vaccine, 40(23), 3263-3271.
- Zhu, F. C., Zhong, G. H., Huang, W. J., Chu, K., Zhang, L., Bi, Z. F., Zhu, K. X., Chen, Q., Zheng, T. Q., Zhang, M. L., Liu, S., Xu, J. B., Pan, H. X., Sun, G., Zheng, F. Z., Zhang, Q. F., Yi, X. M., Zhuang, S. J., Huang, S. J., Pan, H. R., … Xia, N. S. (2023). Head-to-head immunogenicity comparison of an Escherichia coli-produced 9-valent human papillomavirus vaccine and Gardasil 9 in women aged 18-26 years in China: a randomised blinded clinical trial. The Lancet. Infectious diseases, 23(11), 1313–1322. https://doi.org/10.1016/S1473-3099(23)00275-X
- Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, Riveros‐Balta AX, Low N, Henschke N: Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV‐related disease in females and males. Cochrane Database of Systematic Reviews 2019(11).
- Mikamo H, Yamagishi Y, Murata S, Yokokawa R, Han SR, Wakana A, Sawata M, Tanaka Y: Efficacy, safety, and immunogenicity of a quadrivalent HPV vaccine in Japanese men: A randomized, Phase 3, placebo-controlled study. Vaccine 2019, 37(12):1651-1658.
- Lehtinen M, Söderlund‐Strand A, Vänskä S, Luostarinen T, Eriksson T, Natunen K, Apter D, Baussano I, Harjula K, Hokkanen M: Impact of gender‐neutral or girls‐only vaccination against human papillomavirus—Results of a community‐randomized clinical trial (I). International journal of cancer 2018, 142(5):949-958.
- Zizza A, Banchelli F, Guido M, Marotta C, Di Gennaro F, Mazzucco W, Pistotti V, D’Amico R: Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: a systematic review and meta-analysis. Scientific Reports 2021, 11(1):4954.
- Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Hervé C, Descamps D: Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine 2013, 31(48):5745-5753.
- Kojic EM, Kang M, Cespedes MS, Umbleja T, Godfrey C, Allen RT, Firnhaber C, Grinsztejn B, Palefsky JM, Webster-Cyriaque JY et al: Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis 2014, 59(1):127-135.
- WHO. (2022). Human papillomavirus vaccines: WHO position paper (2022 update). Wkly. Epidemiol. Rec., 97, 645-672.
- Review of the current published evidence for single-dose HPV vaccination. (n.d.). PATH. https://www.path.org/our-impact/resources/review-current-published-evidence-single-dose-hpv-vaccination/
- Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, Wagner S, Boland J, Schussler J, Lowy DR et al: Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. JNCI: Journal of the National Cancer Institute 2020, 112(10):1038-1046.
- Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, Bhatla N, Nene BM, Shaw J, Poli URR: Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018, 36(32):4783-4791.
- Barnabas, R.V., Brown, E.R., Onono, M.A. et al. Durability of single-dose HPV vaccination in young Kenyan women: randomized controlled trial 3-year results. Nat Med 29, 3224–3232 (2023). https://doi.org/10.1038/s41591-023-02658-0
- Watson-Jones, D., Changalucha, J., Whitworth, H., Pinto, L., Mutani, P., Indangasi, J., … & Baisley, K. (2022). Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial. The Lancet Global Health, 10(10), e1473-e1484.
- Iversen O-E, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Azurah AGN, Fong SM: Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. Jama 2016, 316(22):2411-2421.
- Phillips, A., Patel, C., Pillsbury, A., Brotherton, J., & Macartney, K. (2018). Safety of Human Papillomavirus Vaccines: An Updated Review. Drug safety, 41(4), 329–346. https://doi.org/10.1007/s40264-017-0625-z.
- Association GOSoCM: Chinese expert consensus on the clinical application of human papillomavirus vaccine. Chinese Journal of Frontier Medicine 2021, 13(2):1-12.





