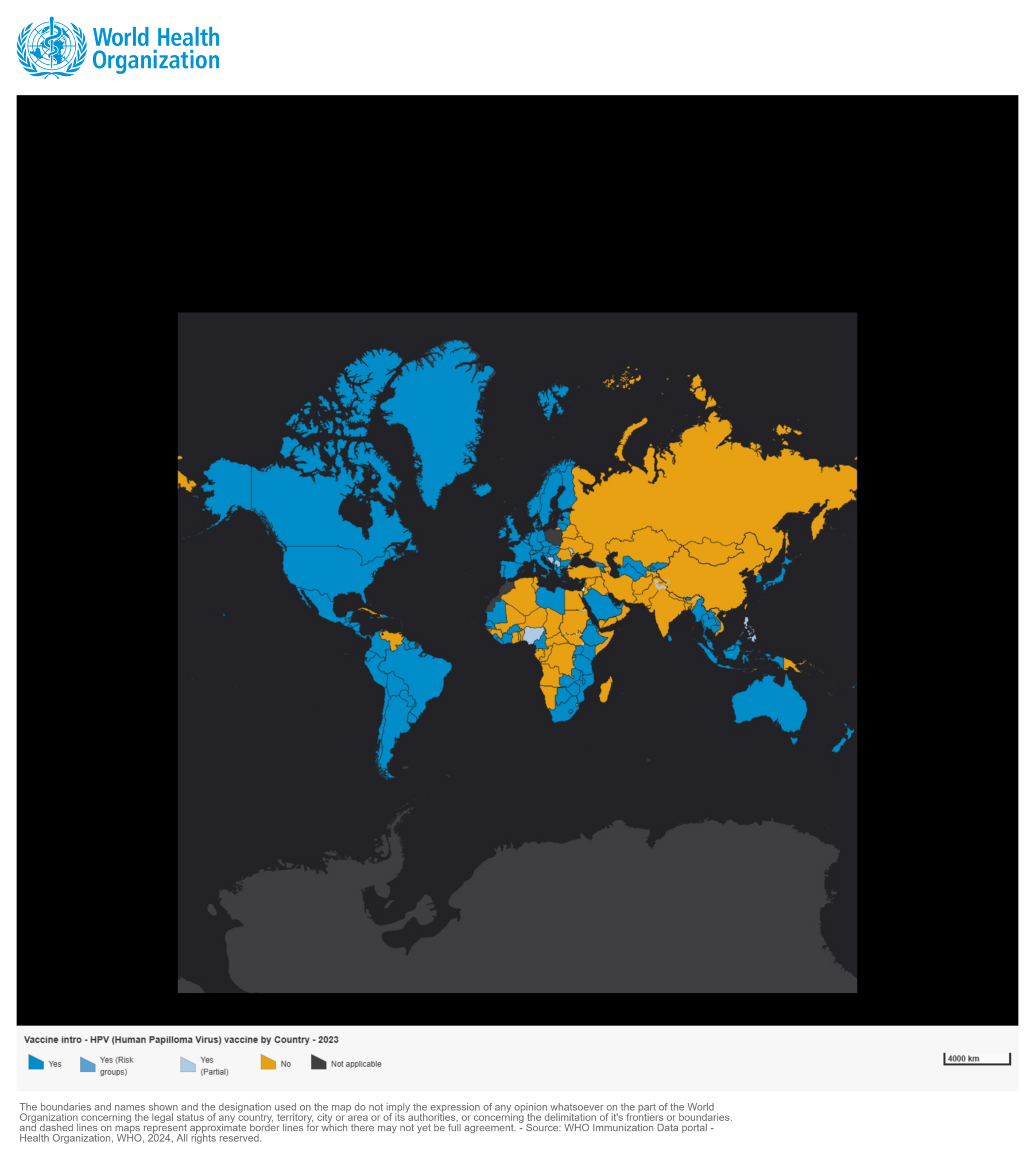
As of December 2023, the WHO and UNICEF monitoring data of the HPV vaccination and coverage showed that among the 194 member countries of the WHO, 140 countries (70%) have introduced the HPV vaccine into their national immunization programs (NIP) [1]. The distribution of these countries is uneven by regions. The American Region (AMRO, approximately 91%) and the European Region (EURO, approximately 85%) showed a higher proportion of countries introduced the HPV vaccine into the NIP, making them the regions with the optimal accessibility. (Figure 1)
Figure 1: Country introduction status of HPV (Human Papilloma Virus) vaccine in the national immunization programme by December 2023
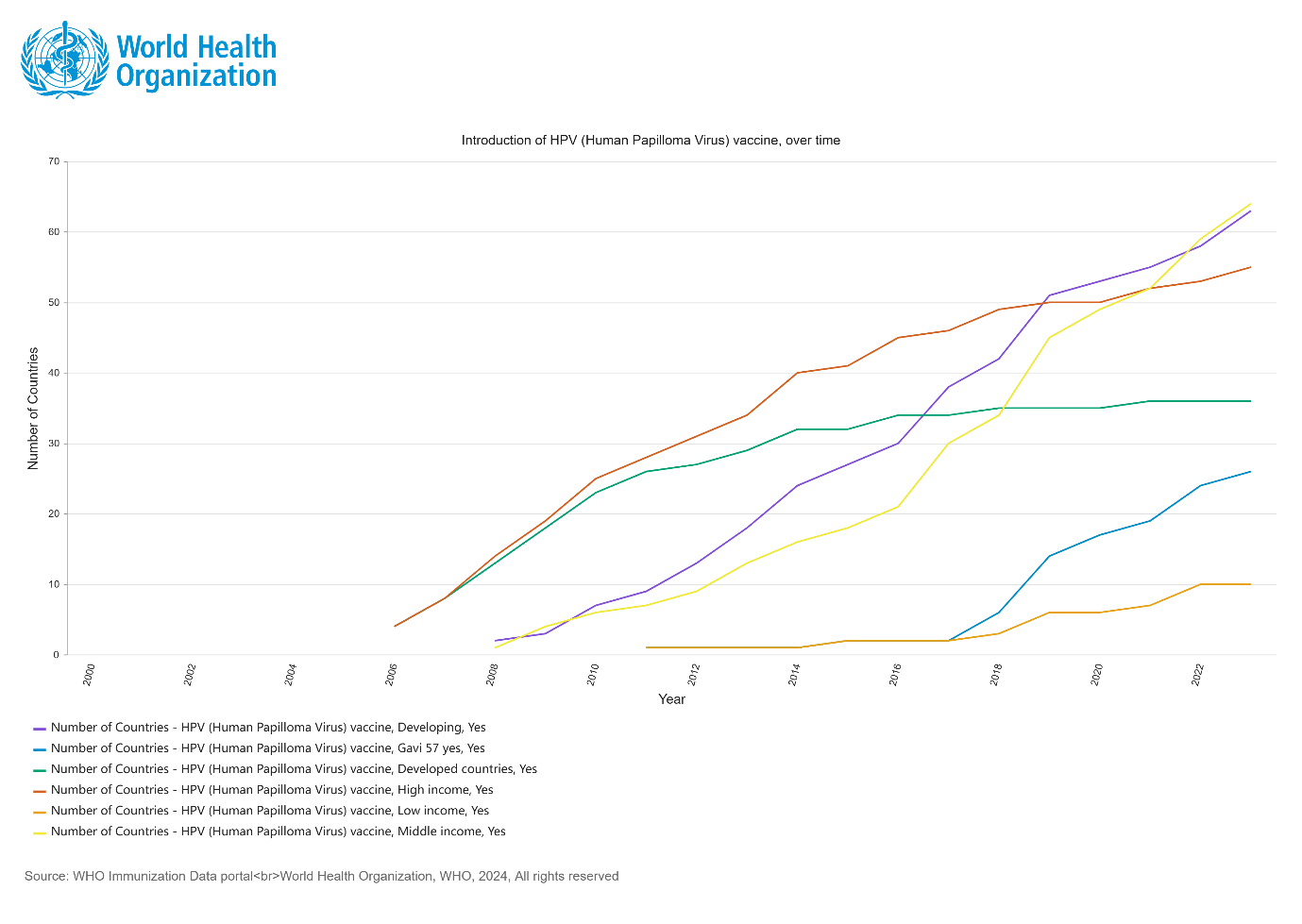
Between 2006 when HPV vaccine was first launched in the market and 2023, 36 high-income countries include HPV vaccine into their national immunization programs. Conversely, developing countries lag behind the progress regarding the late start of the HPV vaccination (from 2009) and the slow progress of introduction. As of 2023, only 10 low-income countries and 64 middle-income countries have included HPV in their national immunization programs.
Since the launch of the WHO Cervical Cancer Elimination Initiative, from 2021 to end of 2023, a further 30 countries introduced the HPV vaccine. Currently, among all LMICs that have included the HPV vaccine in their immunization programs, 26 countries (40%) have received support from Gavi (Figure 2).
Figure 2: Country introduction status of HPV (Human Papilloma Virus) vaccine in the national immunization programme by 2023
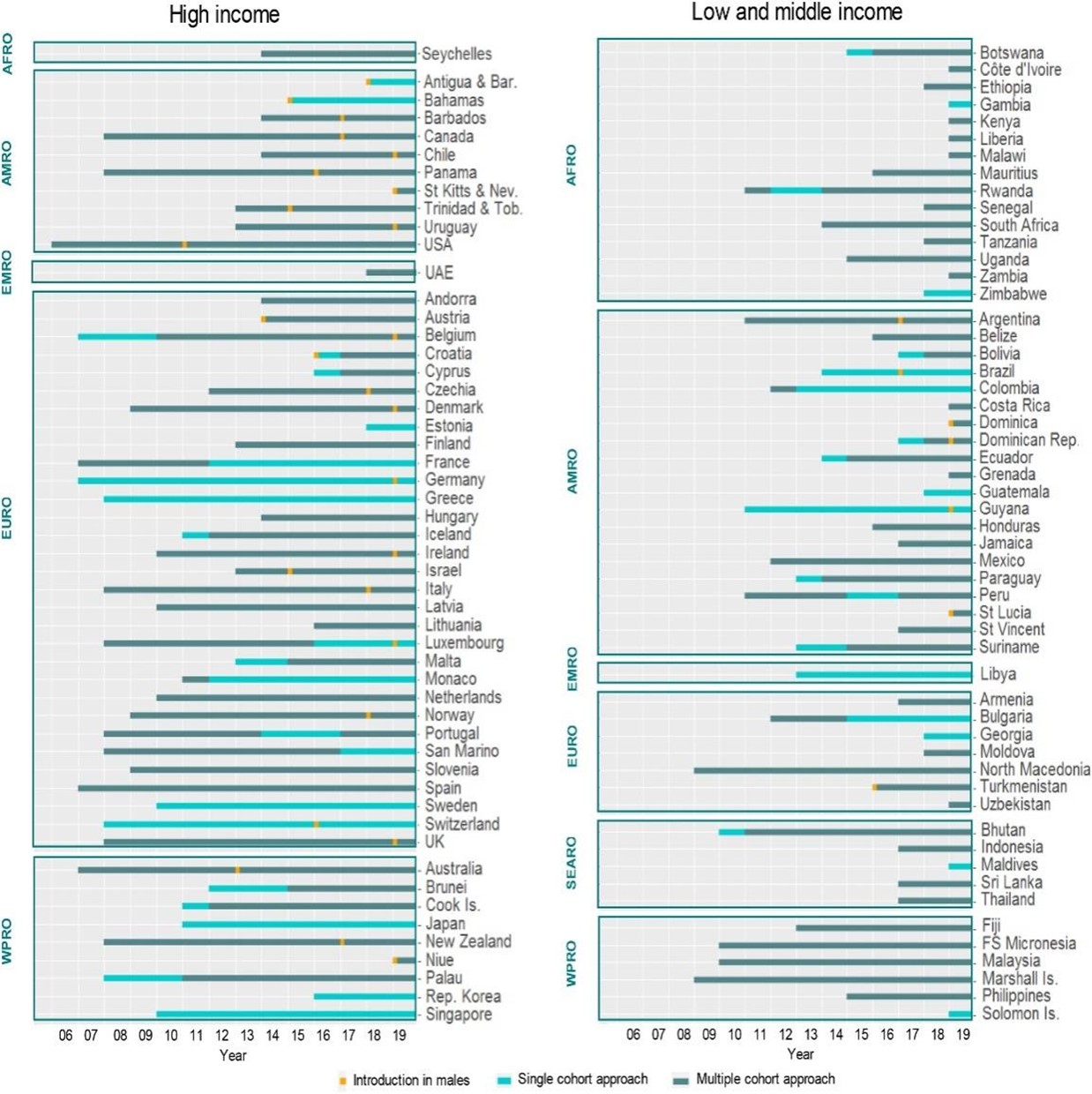
Countries have different HPV vaccination strategies in terms of target population and implementation methods. Table 1 summarizes the vaccine types, target populations, and coverage status of developed countries that have introduced HPV vaccine to the NIP. In LMICs, the primary target population is generally girls aged 9 to 10 or students in specific grades, contrasting with some high-income countries that may extend the target population to girls of 12-26 years old [2]. As of 2023, a total of 57 countries have implemented gender-neutral vaccination strategy, making HPV vaccines equally promoted to eligible boys and girls. Figure 3 shows the target population change in countries where HPV vaccine has been included in the NIP from 2006 to 2019. The shifts in target populations in many low-income countries are related to the scope of Gavi’s funding support. Before 2016, Gavi-funded countries mainly adopted single-cohort vaccination plans. However, since 2016, Gavi-funded countries have implemented multi-age cohort strategies for HPV vaccination, thereby expanding HPV vaccine coverage.
Table 1: Overview and Effectiveness of HPV Immunization Programs in Selected Countries and Regions
| Australia | ||
| Vax | Quadrivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2007.4 | |
| 2007.4 | ||
| 2007.7 | ||
| Status of immunization projects | School-based: girls aged 12-13; Boys aged 12-13 (from Feb 2013) | |
| School-based catch-up: girls aged 14-17 (2007-2009); boys aged 14-15 (2013-2014) | ||
| GP/Community -based catch-up: females aged 18-26 (2007-2009) | ||
| 2 doses <15 years | — | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | School-based: girls aged 15: 86%/84%/78% (2015); boys aged 15: 78%/75%/67% (2015) | |
| School-based catch-up: girls aged 14-15: 84%/80%/74% (2009); girls aged 16-17: 84%/80%/72% (2009) boys aged 14-15: 76%/72%/65% (2014) | ||
| GP/Community -based catch-up: Females aged 18-19: -/-/78% (2009); Females aged 20-26: -/-/56% (2009) | ||
| Effectiveness | Vaccine type-related HPV infection rate: Age 18-26, 3 doses: 86% decline; Age 18-26, 2 doses: 76% decline; Condyloma acuminatum (HPV6/11): 92% decrease CIN/adenocarcinoma: Low-grade CIN: 34% decline; High-grade CIN: 47% decline; Age <20: 54% decline: Age 20-24: 37% decline | |
| Belgium | ||
| Vax | Quadrivalent and bivalent | |
| Quadrivalent and bivalent | ||
| Quadrivalent | ||
| Bivalent | ||
| Cost | Partial funded | |
| Partial funded | ||
| No charge | ||
| No charge | ||
| Vaccine launched date or Immunization program start date | 2007.11 | |
| 2008.12 | ||
| 2010.9 (Flemish community) | ||
| 2011.9 (French community) | ||
| Status of immunization projects | Opportunistic vaccination: girls aged 12-15 | |
| Opportunistic vaccination: girls aged 12-18 | ||
| School-based: girls aged 12-18 | ||
| School-based: girls aged 13-14 | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Opportunistic vaccinations: Girls aged 12-14: -/-/43% (2008-2009); | |
| Girls aged 17: 75%/-/66% (2008-2009) | ||
| School-based: Girls ≤14 : 90%/-/87% (2012) | ||
| School-based: Girls aged 13-14: -/-/29% (2013) | ||
| Canada (Quebec) | ||
| Vax | Quadrivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008 | |
| Status of immunization projects | School-based: Girls aged 9 (2 doses); School-based catch-up: Girls aged 14 (3 doses, 2008-2013) | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | School-based: Girls aged 9: -/78%/- (2012-2013); School-based catch-up: Girls aged 14: -/-/78% (2012-2013) | |
| Canada (Ontario) | ||
| Vax | Quadrivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2007/2008 | |
| Status of immunization projects | School-based: 8th grade girls (approximately 13-14 years old) | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | School-based: Girls aged 9: -/78%/- (2012-2013); School-based catch-up: Girls aged 14: -/-/78% (2012-2013) | |
| Canada (Manitoba) | ||
| Vax | Quadrivalent | |
| Cost | Out of pocket (OOP) | |
| No charge | ||
| Vaccine launched date or Immunization program start date | 2006.8 | |
| 2008.9 | ||
| Status of immunization projects | OOP: Females aged 9-26 | |
| School-based: 6th grade girls (approximately 11-12 years old) | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Voluntary vaccination: Female aged 9-26: -/-/3% (2009) | |
| School-based: Girls aged 11-12: -/-/70% (2011) | ||
| Canada (British Columbia) | ||
| Vax | Quadrivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008.9 | |
| 2015.9 | ||
| Status of immunization projects | School-based: 6th grade girls (approximately 11-12 years old) School-based catch-up: 9th grade girls (14-15 years old) (2008-2011) | |
| High-risk men (age ≤ 26) | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | School-based: 6th grade girls: -/65%/- (2015) School-based catch-up: 9th grade girls: -/-/62% (2011) | |
| — | ||
| Denmark | ||
| Vax | Quadrivalent | |
| Cost | OOP | |
| No charge | ||
| No charge | ||
| No charge | ||
| Vaccine launched date or Immunization program start date | 2006.10 | |
| 2009.1 | ||
| 2008.10 | ||
| 2012.8 | ||
| Status of immunization projects | OOP: Girls and boys aged ≥ 9 | |
| GP Childhood Immunization Programme: Girls aged 12 | ||
| GP catch-up: Girls aged 13-15 (2008-2010) | ||
| GP catch-up: Females aged 20-27 (2012-2013) | ||
| 2 doses <15 years | Started in 2014 | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Females aged 20-27: 46%/35%/2% (2012) | |
| Girls aged 12:-/-/about 90% (2012) | ||
| Girls aged 13-15: 87-90%/83-86%/74-82% (2012) | ||
| Females aged 20-27:-/-/75% (2013) | ||
| Effectiveness | Condylomata acuminata: 2008-2011 women: 67% decline; men aged 15-19: 50% decline [4] CIN: Atypia <18 years old: 33.4% decline 18-20 years old: 12.6% decline CIN2+ 18-20 years old: 14% decline | |
| German | ||
| Vax | Quadrivalent and bivalent (90% Quadrivalent) | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2007.3 | |
| Status of immunization projects | GP/Community: Girls aged 12-17 | |
| Italy (Veneto) | ||
| Vax | Quadrivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008 | |
| Status of immunization projects | Public Health Sector Project: Girls aged 12 | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Girls aged 12: 67-86%/-/56-72% (2017) | |
| Netherlands | ||
| Vax | Bivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2010 | |
| 2009 | ||
| Status of immunization projects | Public Health Sector Project: Girls aged 12 | |
| Public Health Sector Catch-up Project: Girls aged 12-16 (2009) | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Girls aged 13: -/-/61% (2016) | |
| Girls aged 12-16: -/-/52% | ||
| New Zealand | ||
| Vax | Quadrivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008.9 | |
| Status of immunization projects | Schools/GP/Communities: Girls aged 11-12 (from 2009) | |
| School/GP/Community catch-up: Girls aged 18-19 (from 2008); Girls aged 13-17 (2009-2016) | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Girls aged 11-12: -/-/66% (2013) | |
| Girls aged 13-20 (2008-2010): -/-/50% (2012) | ||
| Norway | ||
| Vax | Quadrivalent | |
| Cost | OOP | |
| No charge | ||
| Vaccine launched date or Immunization program start date | 2007 | |
| 2009.8 | ||
| Status of immunization projects | — | |
| School based: Girls aged 12 | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | — | |
| Girls aged 12: 70-83%/-/68-76% (2013) | ||
| Spanish | ||
| Vax | Bivalent | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008 | |
| Status of immunization projects | Basic medical services: Girls aged 14 | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Girls aged 14: -/-/72% (2013) | |
| Sweden | ||
| Vax | Quadrivalent | |
| Cost | Partially subsidized | |
| No charge | ||
| Vaccine launched date or Immunization program start date | 2006.1 | |
| 2012 | ||
| Status of immunization projects | Opportunistic vaccination: Girls aged 13-20 | |
| School-based: Girls aged 10-12; School-based catch-up: girls aged 13-18 | ||
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Girls aged 13-20: -/-/25-30% (2006-2011) | |
| School based: Girls aged 10-12: 80%/75%/- (2016) School-based catch-up: Girls aged 13-18: -/-/60% (2013) | ||
| United Kingdom | ||
| Vax | Bivalent 2008-2012; Quadrivalent 2012 | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008.9 | |
| Status of immunization projects | School-based: Girls aged 12-13 Schools/GP catch-up: Girls aged14-17 | |
| 2 doses <15 years | Started in 2014.9 | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | School-based Project Girls aged 12-13: 91%/90%/88% (2013/14) School-based catch-up: Girls aged 14-17: -/-/56% (39-76%) (2011) | |
| Effectiveness | Vaccine type-related HPV infection rate: 16-18 year old: HPV16/18 infection rate decreased from 19.1% to 6.5% Condyloma acuminatum (HPV6/11): Bivalent: 20.8% decline Quadrivalent: 38.9% decline in women and 30.2% decline in men | |
| United Kingdom (Scotland) | ||
| Vax | Bivalent 2008-2012; Quadrivalent 2012 | |
| Cost | No charge | |
| Vaccine launched date or Immunization program start date | 2008.9 | |
| Status of immunization projects | School-based: Girls aged 12-13; Schools/GP catch-up: girls aged 14-17 (2008-2011) | |
| 2 doses <15 years | Started in 2014 | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | School-based: Girls aged 12-13: -/-/90% (2011) Schools/GP catch-up: Girls aged 13-17: -/-/88% (33% of school leavers) (2011) | |
| Effectiveness | Vaccine type-related HPV infection rate: Decline from 29.8% to 13.6% CIN: 29% decline in CIN1 50% decline in CIN2 55% decline in CIN3 | |
| United States of America | ||
| Vax | Quadrivalent: 2006-2016; Nine-valent: 2016.12 | |
| Cost | No charge + OOP | |
| Vaccine launched date or Immunization program start date | 2006.6 | |
| Status of immunization projects | Basic medical services: Girls aged 11-12: routine vaccination; Girls aged 13-26: catch-up; Boys aged 11-12: routine vaccination; Boys aged 13-21: catch-up (from 2011) MAM/immunosuppressed people aged 22-26 | |
| 2 doses <15 years | Started in 2016.10 | |
| Vax coverage: ≥1 dose/2 doses/3 doses (annual) | Routine and catch-up vaccinations: Girls aged 13-17: 60%/50%/40% (2014) Boys aged 13-17: 42%/31%/22% (2014) Females aged 19-26: 42% took at least 1 dose (2015) Males aged 19-26: 10% took at least 1 dose (2015) | |
| Effectiveness | Vaccine type-related HPV infection rates: 56% decline in female aged 14-19 (2010) 64% decline in female aged 14-19 (2012) 34% decline in female aged 20-24 (2012) Condyloma acuminatum (HPV6/11): <21 years old: 34.8% decline >21 years old: 10% decline HPV16/ 18CIN2+: 33% decline | |
The implementation methods also differ across countries. Approximately 59% of HPV vaccination programs rely on school mobilization as the primary strategy or collaborate with medical institutions to vaccinate students. In high-income countries, the proportion of programs led by schools and medical institutions is relatively equal. In contrast, over 90% of programs in LMICs use a school-based approach to administered the HPV vaccine [2]. In some LMICs, such as Malaysia, South Africa, Sri Lanka, Uganda, Zambia, etc., school-based HPV vaccination programs have become routine school activities or been integrated with other school events, such as Children’s Health Day. In Latin America, HPV vaccination programs are integrated with national immunization weeks or other youth health activities [3].
Figure 3. Changes over time in national HPV programs (eligibility criteria)
Content Reviewer: Kelly Hunter, Tianyi Deng
Page Editor: Jiaqi Zu
References:
- World Health Organization. (2023, November 17). Global partners cheer progress towards eliminating cervical cancer and underline challenges. Www.who.int. https://www.who.int/news/item/17-11-2023-global-partners-cheer-progress-towards-eliminating-cervical-cancer-and-underline-challenges
- Gallagher KE, LaMontagne DS, Watson-Jones D: Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018, 36(32):4761-4767.
- Tsu VD, LaMontagne DS, Atuhebwe P, Bloem PN, Ndiaye C: National implementation of HPV vaccination programs in low-resource countries: Lessons, challenges, and future prospects. Preventive medicine 2021, 144:106335-106335.
- Sandø N, Kofoed K, Zachariae C, Fouchard J. A reduced national incidence of anogenital warts in young Danish men and women after introduction of a national quadrivalent human papillomavirus vaccination programme for young women–an ecological study. Acta Derm Venereol. 2014 May;94(3):288-92. doi: 10.2340/00015555-1721. PMID: 24150529.





