Expanding the coverage of combination vaccines in China faces multiple obstacles. One of the barriers is the conflict with the existing immunization schedule, which can be divided into two major issues. The first is the challenge of including the Haemophilus influenzae type b (Hib) vaccine or combination vaccines containing Hib antigen in the National Immunization Program (NIP). Secondly, introducing combination vaccines containing Hepatitis B antigen conflicts with the current monovalent Hepatitis B vaccine schedule.
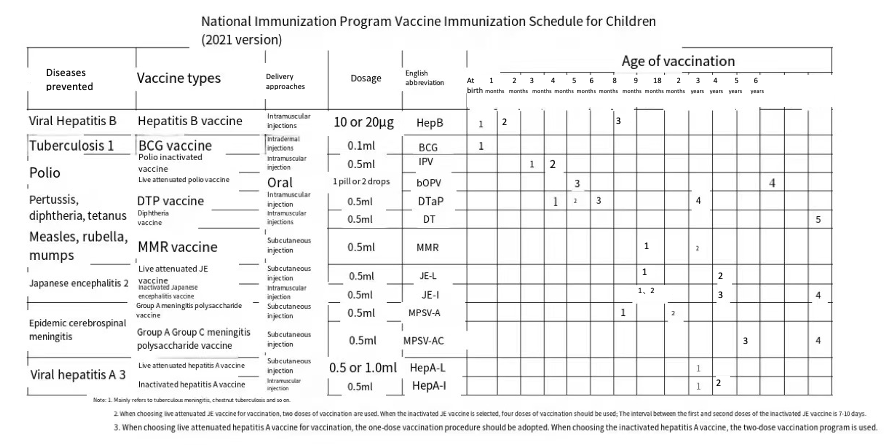
Since its inception in 1978, China’s NIP has evolved from targeting six diseases with four vaccines to covering fifteen diseases with fourteen vaccines. However, since 2007, the NIP has not expanded by adding new types of vaccines. Four vaccines recommended by the World Health Organization (WHO) to be included in all member countries’ immunization programs (HPV, RV, PCV, and Hib) have not yet been introduced into China’s NIP. Among the 194 WHO member states, 99% have included the Hib vaccine in their immunization programs, with China being the only WHO country that has not yet included Hib in its NIP1.
Data source: https://www.chinacdc.cn/nip/kyjz/mycxbjsm/mycxb/202105/t20210513_230543.html
There are two main categories of non-NIP vaccines in China. The first category is alternative non-NIP vaccines, which have different characteristics or vaccination schedules from NIP vaccines. For example, the quadrivalent and pentavalent vaccines can prevent the same vaccine-preventable diseases (VPDs) covered by the NIP vaccines, such as diphtheria, tetanus, pertussis, and polio, with additional protection against Hib-related diseases. The second category is complementary non-NIP vaccines, utilized to prevent diseases not yet included in the NIP. The monovalent Hib vaccine falls into this category 2.
In the past, the National Immunization Advisory Committee (NIAC) has proposed prioritizing the inclusion of key vaccines, including the Hib vaccine, into the NIP. However, this decision requires considering multiple factors, including the local disease burden, financing capacity, and affordability for residents. The evidence-informed decision-making process has progressed slowly, which has hindered the expansion of the coverage of high-valent pediatric combination vaccines3.
Including combination vaccines (with HepB antigen) into the NIP faces a different challenge: the conflict between its administered schedule and the monovalent HepB vaccine already included in the NIP.
Multiple studies and clinical guidelines have confirmed that timely administration of the first dose of monovalent hepatitis B vaccine to newborns is crucial for interrupting mother-to-child transmission of the hepatitis B virus (HBV)4,5,6. China introduced the monovalent hepatitis B vaccine in 1985, and with the increase in vaccine coverage, the national hepatitis B infection rate has gradually decreased 7. After the introduction of the HepB vaccine into the NIP, the prevalence of chronic HBV infection among children under five years old dropped from 9.7% to less than 1%8. This underscores the importance of timely monovalent HepB vaccination for newborns.
The monovalent HepB vaccine in the current immunization program is administered in three doses (at birth, 1 month, and three months). The high-valent combination vaccine (e.g., the hexavalent vaccine, DTaP-Hib-IPV-HepB) has a more flexible schedule with three primary doses being administered at 6 weeks, 10 weeks, and 14 weeks or 2 months, 3 months, and 4 months. Therefore, introducing the high-valent combination vaccine requires further studies to evaluate the impact of delaying the delivery of the second dose on the immunogenicity and disease control, the safety of receiving multiple doses of HepB vaccines, and the alternate use of monovalent vaccines and the combination vaccines containing HepB antigen.
Studies in other countries demonstrated that the delayed administered second dose of the hepatitis B vaccine did not affect the immunogenicity of the vaccine but seemed to increase the level of hepatitis B surface antibodies (anti-HBs) to a certain extent9,10,11,12. Further studies in China had shown that delaying the second dose of hepatitis B vaccine for up to 60 days did not affect the level of hepatitis B surface antibodies (anti-HBs) and the positivity rate, even if the mothers were mono-positive or double-positive for hepatitis B surface antigen (HBsAg) and/or hepatitis B E antigen (HBeAg)13,14.
In response to the second point, the hepatitis B vaccine is inactivated and has a superior safety profile15. Overseas studies and WHO position papers indicated that the risk of a significant increase in reactogenicity after receiving additional doses or doses of the Hepatitis B vaccine is minimal and that the human body will not suffer any harm as a result16,17.
Regarding introducing the high-valent combination vaccines with hepatitis B antigen in conflicts with the immunization schedule, other countries’ practices are to take both the hepatitis B monovalent vaccine and the combined hepatitis B-containing vaccine after the newborns assessed by pediatricians as being at risk of hepatitis B virus infection. In the United Kingdom, most babies born to mothers infected with hepatitis B will receive a total of 6 doses of vaccine to prevent hepatitis B virus infection between birth and 12 months of age. Three doses are part of the hexavalent vaccine covered by the immunization schedule, administered at 8, 12, and 16 weeks of age. Additionally, these infants will receive three doses of monovalent Hepatitis B vaccine at birth, 4 weeks, and 12 months of age, respectively18. Suppose an infant is born to a hepatitis B-negative mother but will be living with another hepatitis B-infected person and is at immediate risk for infection. In that case, the infant will require a single dose of hepatitis B monovalent vaccine before discharge from the hospital and follow the routine immunization schedule beginning at 8 weeks18.
The WHO 2017 position paper for the hepatitis B vaccine recommends alternating hepatitis B monovalent vaccines with hepatitis B-containing combination vaccines. The WHO recommends that if a three-dose regimen is chosen, one dose of monovalent vaccine can be administered at birth. The second and third doses of the Hepatitis B monovalent vaccine or hepatitis B-containing combination vaccine can be co-administered with the first and third doses of the DTP-containing vaccines. Furthermore, suppose a four-dose regimen is selected for programmatic reasons. In that case, the one-dose monovalent vaccine is given at birth and followed by 3-doses of Hepatitis B monovalent vaccines or hepatitis B-containing combination vaccines, administered during the same visits as the three doses of DTP-containing vaccines17.
Before July 2022, early regulations in China were not conducive to inter-enterprise collaboration in developing combination vaccines. The China National Medical Products Administration (NMPA) issued the “Rules of Vaccine Manufacturing and Distribution” in July 2022, which provided favorable regulations for the joint development of combination vaccines. However, compared with counterparts in Western countries, the rules are less flexible and more sophisticated in practice. No joint development business case has been initiated since the issuance of the regulations.
The Marketing Authorization Holder (MAH) system is widely adopted by the pharmaceutical industry in developed countries. It emphasizes the legal responsibility of the MAH in the life course of the drug, which is easier for integrated management. Adopting the MAH system allows research institutions and natural persons without corresponding production qualifications to obtain qualifications through collaborative R&D or contract manufacturing. It advances drug R&D innovation and resource allocation optimization and improves administrative supervision.
China’s MAH system was first proposed in August 2015. In the second half of 2015, a series of policies and regulations related to the MAH system were introduced. It was formally written into the 2019 Drug Administration Law and implemented nationwide on December 1st, 201919.
Before this, the MAH system was implemented for many years in Europe, the United States, Japan, etc. Europe and the United States implement a system in which the marketing authorization and the manufacturing authorization are independent, allowing the applicant and the holder of the marketing authorization to be different subjects. China has different regulations. Although the Drug Administration Law in China separated the manufacturing license and marketing authorization license, the applicant and the holder should still need to be the same20.
Referring to China’s Drug Administration Law and Vaccine Administration Law, China’s MAH system also sets certain restrictions on the applicants. Individuals are not eligible to apply for marketing authorization. Only enterprises or research institutes with relevant product licenses can apply for the MAH. The difference is that the MAH in the EU does not have any restrictions on the applicant subjects, and both individuals and companies can apply. Meanwhile, authorized product distributors in the EU are also eligible21. The United States has even more flexible requirements for applicants; individuals, enterprises, government agencies, academic institutions, associations, private organizations, or other organizations are all eligible to apply21.
In China, the national authority must approve the commissioned manufacturing of vaccines. After the commissioned entity is identified, it cannot be recommissioned again. On the other hand, the MAH in the EU and the U.S. has no restriction on product type. There is no need to go through a special approval process when commissioning the manufacturing of vaccines. Therefore, in Europe, the United States, and other regions, one or more vaccine approval numbers held by the same MAH can be produced by multiple manufacturers (manufacturers can be in different countries), and different vaccine MAHs can collaborate to develop combination vaccines.
In Europe, when a vaccine passes the marketing authorization review, the EU will issue a Vaccine Antigen Master File (VAMF) certificate, significantly simplifying the process required to place a vaccine on the market22. The VAMF is an independent document required when applying for vaccine marketing authorization. It contains all the information on the biological, chemical, and pharmacological properties of each active substance in the vaccine. A vaccine may contain one or more active substances of vaccine antigens. An independent VAMF can be used when applying for the same monovalent or combination vaccines. When developing a new type of vaccine, if some vaccine antigen components of the combination vaccine are identical to those of marketed vaccines, the EU regulation stipulates that research institutions can directly submit the previously awarded VAMF certificate. If the vaccine under development contains new active substances, the applicant must provide the authorities with a marketing authorization application document for the new active substance.
In the United States, the Food and Drug Administration (FDA) has a Center for Biologics Review and Research (CBER), which is responsible for assuring the safety and efficacy of biological products. New biological products, including vaccines, need to go through an application review process similar to that for pharmaceuticals before they launch in the market. However, CBER allows for a “case-by-case” approach to discussing with applicants the use of technical information on marketed ingredients in combination vaccine applications23.
Some studies point out that combination vaccines are manufactured using an individual purification process in developed countries, whereas, in China, a co-purification process is used in the production of the DTP combination vaccine. Thiomersal may be added as a preservative or inactivating agent during the manufacturing process, though the use of thiomersal can destroy the active ingredient in the polio vaccine24.
Caregivers and vaccine recipients
A study reveals that Chinese parents are generally less aware of non-NIP vaccines except influenza and varicella. Among the nine non-NIP vaccines included in the study , the awareness of the pentavalent vaccine among interviewed Chinese parents is the lowest, with less than 20%25. Additionally, researchers specifically collected information on the pentavalent vaccine in Dongcheng District, Beijing. Of 183 surveyed caregivers of the 1-month-old infants, only 39 had heard about the pentavalent vaccine, accounting for 21.31%. Furthermore, only 10.93% of the interviewed parents clearly understood which NIP vaccines could be replaced by the pentavalent vaccine26.
A cross-sectional survey conducted in 2024 examined the acceptance and willingness to pay for the DTaP-HBV-IPV-Hib hexavalent vaccine among Chinese parents. A total of 581 parents of children aged 0-6 years from seven cities in China participated in the survey. The study revealed that between April 28, 2023, and June 30, 2023, 435 out of 581 parents (74.87%, 95% CI: 71.3%-78.4%) expressed acceptance of the hexavalent vaccine. The primary factors influencing parents’ vaccination decisions included place of residence, education level, experience with paying for vaccination, and disease knowledge score. The mean willingness to pay for the full immunization (four doses) was 2266.66 yuan (SD = 1177.1), with a median of 2400 yuan (IQR: 1600-2800). The child’s age (p < 0.001), parental education level (p = 0.024), and perceived price barriers (p < 0.001) were significantly associated with willingness to pay. Overall, parents showed high acceptance and willingness to pay for the hexavalent vaccine. If the out-of-pocket expenses for parents are reduced, their willingness to accept the vaccine may increase. Therefore, including the vaccine in medical insurance coverage, free government provision, or reduction in its price could potentially enhance parental acceptance27.
In collecting and consolidating information, we have also identified some evidence gaps that pose challenges to implementing evidence-informed decision-making for policymaking. We listed them below:
- High-quality data on the disease burden of the VPDs (incidence, morbidity, and mortality) in different regions of China
- The accuracy of available data related to the burden of disease needs to be improved, owing to underreporting, weak surveillance, lack of guidance, and inconsistent case definitions
- Supply, demand, and vaccination coverage of combination vaccines in different regions of China
- Existing evidence was conducted a long time ago, has small sample sizes, and lacks analysis of regional variability
- Analysis of awareness, knowledge, willingness to vaccinate, and factors influencing the use of pediatric combination vaccination
- Existing studies have a small sample size. The available evidence included the broad category of non-NIP vaccines, lacking research specifically for high-valent combination vaccines.
- Vaccine-related research
- Existing studies have a small sample size. The available evidence included the broad category of non-NIP vaccines, lacking research specifically for high-valent combination vaccines.
- No pilot immunization program is related to high-valent pediatric combination vaccines.
- Evaluation of effectiveness: compare high-valent combination vaccines with traditional monovalent or lower-valent vaccines; compare combination vaccines with different carriers.
- Lack of studies related to hexavalent vaccine
- Lack of comparative studies on the effectiveness of domestic and imported vaccines
- Study on immunization schedules: resolving conflicts in immunization schedules
- There has been no discussion on resolving the schedule conflicts between the NIP and the combination vaccines.
- Monitoring the simultaneous administration of combination vaccine and other vaccines
- There is a lack of research on the simultaneous administration of combination vaccines and other NIP vaccines or commonly used non-NIP vaccines.
- Existing studies have a small sample size. The available evidence included the broad category of non-NIP vaccines, lacking research specifically for high-valent combination vaccines.
- Study of key intervention strategies to improve the use of combination vaccination
- Strategies for promoting combination vaccines from an interdisciplinary perspective (including clinical medicine, epidemiology, sociology, psychology, etc.)
Content Editor: Menglu Jiang
Page Editor: Ziqi Liu
Bibliography
1 Wang N, Li Q, Li JH, Wang YM, Ma CH, Zheng CJ, Yin ZD. Status of vaccine integration into national immunization programs in 194 WHO member countries[J]. China Vaccine and Immunization,2021,27(2):214-220.
2 Wang, W.C. et al. An analysis of the current situation and factors affecting non-immunization vaccination in China. China Vaccine and Immunization. 2020, Issue 1.
3 Ma, C., Li, J., Wang, N., Wang, Y., Song, Y., Zeng, X., Zheng, C., An, Z., Rodewald, L., & Yin, Z. (2022). Prioritization of Vaccines for Inclusion into China’s Expanded Program on Immunization: Evidence from Experts’ Knowledge and Opinions. Vaccines, 10( 7), 1010. https://doi.org/10.3390/vaccines10071010
4 Xia G-L et al. Evaluation of the protective effect of a recombinant hepatitis B vaccine program to interrupt mother-to-child transmission of hepatitis B virus. Chinese Journal of Epidemiology. 2003,24(5):362-364,365
5 Chinese Medical Association et al. Guidelines for primary care of chronic hepatitis B (2020). Chinese Journal of General Practice. 2021,20(2): 137-149. DOI: 10.3760/cma.j.cn114798-20201218-01262
6 Chinese Medical Association, Division of Perinatal Medicine. Clinical guidelines for the prevention of mother-to-child transmission of hepatitis B virus (2020). Journal of Clinical Hepatobiliary Diseases. 2020, 36(7): 1474-1481. DOI: 10.3760/cma.j.cn112141-20200213-00101.
7 Cui, F., Shen, L., Li, L., Wang, H., Wang, F., Bi, S., Liu, J., Zhang, G., Wang, F., Zheng, H., Sun, X., Miao, N., Yin, Z., Feng, Z., Liang, X., & Wang, Y. ( 2017). Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. emerging infectious diseases, 23(5), 765-772 . https://doi.org/10.3201/eid2305.161477
8 Liang, X., Bi, S., Yang, W., Wang, L., Cui, G., Cui, F., Zhang, Y., Liu, J., Gong, X., Chen, Y., Wang, F., Zheng, H., Wang, F., Guo, J., Jia, Z., Ma, J., Wang, H., Wang, H. (2009.). , Luo, H., Li, L., Jin, S., … Wang, Y. (2009). Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine, 27(47), 6550- 6557. . 6557. https://doi.org/10.1016/j.vaccine.2009.08.048
9 Hadler, S. C., de Monzon, M. A., Lugo, D. R., & Perez, M. (1989). Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine, 7(2), 106-110. https://doi.org/10.1016/ 0264-410x(89)90046-7
10 Middleman, A. B., Kozinetz, C. A., Robertson, L. M., DuRant, R. H., & Emans, S. J. (2001). The effect of late doses on the achievement of seroprotection and antibody titer levels with hepatitis b immunization among adolescents. pediatrics,. 107(5), 1065-1069. https://doi.org/10.1542/peds.107.5.1065
11 Huang, Jiemei et al. Study on the inclusion of hepatitis B vaccine in the planned immunization program. Chinese Journal of Epidemiology. 1992,13(1):1-4.
12 Xiao M. Study on the immunization effect of high-dose hepatitis B vaccine and its influencing factors in newborns in Beijing. Peking Union Medical College Chinese Academy of Medical Sciences Tsinghua University School of Medicine. 2015.
13 Yang JY et al. Study on the inclusion of hepatitis B vaccine in different immunization procedures of planned immunization (EPI)[J]. Guangxi Medicine. 1994, 16(3): 281-283.
14 Chen Huifeng et al. Study on the relationship between the timing of the 2nd vaccination and the immunization effect of hepatitis B vaccine in newborns[J]. Chinese Journal of Epidemiology. 1994, 14(6): 349-350.
15 Lin et al. Comparison of the Safety of Hepatitis B Vaccine Types I and II in Fujian Province, 2015-2016. Practical Preventive Medicine. 2018(25). Issue (1): 34-36.
16 Marcy S. M. (2003). Pediatric combination vaccines: their impact on patients, providers, managed care organizations, and manufacturers. the American journal of managed care, 9(4), 314-320.
17 World Health Organization. Hepatitis B vaccines: WHO position paper. Weekly Epidemiological Record, 2017, vol. 92.27.
18 University of Oxford. Vaccine Knowledge: 6-in-1 vaccine key facts. Available online: https://vaccineknowledge.ox.ac.uk/6-in-1-vaccine#Key- Accessed 20 March 2023.
19 Han, Chacha et al. Study on the Implementation of Drug Listing License Holder System and Countermeasures in China. Chinese Journal of New Drugs. 2019, Issue 5. 593-597.
20 Xie, Jinping et al. Impact of the Marketing Authorization Holder (MAH) System on the Current Regulatory System and Suggestions on the Interface. China Health Policy Research. 2018 (11). Issue (12): 1-6. DOI: 10.3969/j.issn. 1674-2982. 2018.12.001
21 Bao Peng et al. Comparison of drug registration in China, the United States and Europe: MAH system integration and empowerment. http://m.cnpharm.com/c/2020-09-29/757774.shtml
22 The European Agency for the Evaluation of Medicinal Products (2005). Guideline on requirements for vaccine antigen master file (VAMF) certification. Available online: https://www.ema.europa.eu/en/documents/ scientific-guideline/guideline-requirements-vaccine-antigen-master-file-vamf-certification_en.pdf. Accessed 21 March 2023.
23 U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research (1997). Guidance for industry for the evaluation of combination vaccines for preventable diseases: production, testing and clinical studies. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-evaluation-combination-vaccines- preventable-diseases-production-testing-and. Accessed 10 June 2023.
24 Sawyer, L. A., McInnis, J., Patel, A., Horne, A. D., & Albrecht, P. (1994). Deleterious effect of thimerosal on the potency of inactivated poliovirus vaccine. Vaccine, 12(9), 851-856. https://doi.org/10.1016/ 0264-410x(94)90296-8
25 Zhong, Xiaoling et al. A survey on children’s parents’ knowledge, beliefs and behaviors regarding the second type of vaccine. Frontiers of Medicine. 2017 (12) Vol. 35. doi: 10.3969/j.issn.2095-1752.2017.35.321
26 Yan Wei et al. A Survey on Knowledge of Immunization against Type II Vaccines in Dongcheng District, Beijing. Capital Public Health. 2014(8). No. 4.





