Early data show that as of October 2012, 114 countries globally had included pentavalent vaccines in their immunization programs1. According to the World Health Organization and United Nations Children’s Fund estimates for country-level immunization coverage (WUENIC), the number of countries that have included pediatric pentavalent vaccines had grown to 132 in 2017-20182. By 2020, with the support of Gavi, 73 low-income countries had introduced the pentavalent vaccine2. In recent years, some countries have begun to use the hexavalent vaccine to replace the pentavalent vaccine. According to a study, by 2019, the hexavalent vaccine had been marketed and used in over 100 countries worldwide, with 35 countries introducing it into their immunization programs3.
The Global Alliance for Vaccines and Immunization (Gavi) has been providing combination vaccine for children in the world’s least developed countries since 2001. In 2000, only less than 1% of children in the 67 countries Gavi-eligible countries had been vaccinated with pentavalent vaccines. In 2019, pentavalent vaccines reached a full coverage rate of 82% of school-age children in those countries, with declining slightly to 78% and 77% in 2020 and 2021. By the end of 2021, more than 661 million children from Gavi-eligible countries had received pentavalent vaccines4.
In November 2018, the GAVI Secretariat pre-approved a support program for hexavalent vaccines, with the aim of facilitating efforts to eradicate polio and further enhance global coverage of combination vaccines.
There are different strategies to mitigate the VPDs’ disease burden. The combination vaccines included in the National Immunization Programs (NIP) vary in different countries. We outlined the coverage and types of vaccines targeting diphtheria, tetanus, pertussis, measles, mumps, rubella, hepatitis B, Hemophilus influenzae type b, and varicella in selected case countries. Many low- and middle-income countries obtain the pentavalent vaccine through Gavi, while developed countries predominantly use high-valent combination vaccines for childhood immunization programs. For example, in European countries like the United Kingdom, France, and Germany, the hexavalent vaccine has been prioritized in their childhood immunization schedules.
Types and coverage of selected pediatric vaccines among 0-6 years old children against DPT, MMR, hepatitis B,Haemophilus influenzae type b (Hib) and varicella in selected case countries
Case country 1: United Kingdom
The British immunization schedule includes hexavalent vaccines (DTaP-Hib-HepB-IPV) with first, second, and the third primary dose given at 2, 3 and 4 months of age and a booster dose using DTaP-IPV quadrivalent vaccine at 3 years old. The United Kingdom offers monovalent Hepatitis B vaccine to high-risk children who were born to mothers infected by Hepatitis B virus. The administration schedule has three additional doses of monovalent Hepatitis B vaccine at birth, 4 months, and 12 months. The varicella vaccine is not part of the UK Childhood Immunization Program and is only offered to those who are at high risks and have close contact to people with varicella.
Selected vaccines and immunization schedule
| Types of Vaccine | Vaccination Schedule | Note | No. of doses | ||||||
| At birth | 2 months | 3 months | 4 months | 13 months | 1 year old | 3 years old | 6-9 doses (depends on different vaccines and risk groups) | ||
| DTaP-Hib-HepB-IPV | first dose | second dose | third dose | ||||||
| DTaP-IPV | first dose | ||||||||
| MMR | first dose | second dose | |||||||
| HepB (Monovalent) | first dose | second dose | third dose | Available to babies born to hepatitis B infected mothers in addition to the three doses of hexavalent vaccine. | |||||
Information source: https://immunizationdata.who.int/global?topic=Vaccination-schedule&location=GBR
According to 2021-2022 National Health Service (NHS) statistics, the coverage rate of the hexavalent vaccine (DTaP-Hib-HepB-IPV) among the British infants aged 12 months was 92.3%, with the highest in Scotland at 96.3%, and in the Welsh, Northern Ireland and England were 95.2%, 93.5% and 91.8% respectively5. Coverage of the hexavalent vaccine (DTaP-Hib-HepB-IPV) was 93.5% among British infants aged 24 months, 97.1% in Scotland, and 96.3%, 95.3%, and 93.0% in Wales, Northern Ireland, and England respectively.
After September 2017, the pentavalent vaccine (DTaP-IPV-Hib) in the British National Immunization Program (NIP) were replaced by the hexavalent vaccine. Additional protection against hepatitis B infection was provided for all infants born after August 1st , 2017. According to data disclosed by the NHS from 2021 to 2022, among the under-5-years British infants, the coverage of the first dose pentavalent vaccine is 94.8%, with the highest in Scotland at 97.4%, followed by Northern Ireland at 96.7%, and 96.4% and 94.4% in Wales and England5.
Coverage of the pre-school DTaP-IPV booster shot reached 85.3% among children aged 5 years, with Scotland continuing to have the highest coverage rate at 92.7%, followed by Wales at 91.2%, Northern Ireland at 89.7% and England at 84.2%.
First dose MMR vaccine reached 93.8% among 5 years old children, with coverage of over 95% in Scotland, Wales and Northern Ireland, and the proportion of young children with full course immunization (including two doses) was 86.5%. The coverages in Scotland and Wales are over 90%, with Northern Ireland close to 90% and England only 85.7%.
Case country 2: France
The French immunization program includes hexavalent vaccine (DTaP-Hib-HepB-IPV) with different immunization schedule. The first dose is given at 2 months followed by the second and third dose at 4 and 11 months respectively. DTaP-IPV quadrivalent vaccine is given at 6 years old as booster dose. Similar to the United Kingdom, the hepatitis B monovalent vaccine is only offered to newborns who are at risk of hepatitis B infection through mother-to-child transmission.
Selected vaccines and immunization schedule
| Type of vaccine | Vaccination Schedule | Note | No. of doses | ||||||
| At birth | 2 months | 3 months | 11 months | 12 months | 18 months | 6 years old | 6-7 (depends on different vaccines and risk groups) | ||
| DTaP-Hib-HepB-IPV | first dose | second dose | third dose | ||||||
| DTaP-IPV | first dose | ||||||||
| MMR | first dose | second dose | |||||||
| HepB | first dose | Available only to infants born to mothers infected with hepatitis B at birth to interrupt mother-to-child transmission | |||||||
Data source: https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-country_name?DISEASECODE=HEPATITISB&TARGETPOP_GENERAL=
According to the Statista, in 2018, the coverage of the first dose hexavalent vaccine among eligible children in France reached 96.4%. The full-dose vaccination rate (three doses) was 84.1%. In 2019, the first dose coverage reached to 99.1% and the full vaccination rate was 90.3%. The two indicators reached to 99.4% and 90.5% respectively in 2020 6.
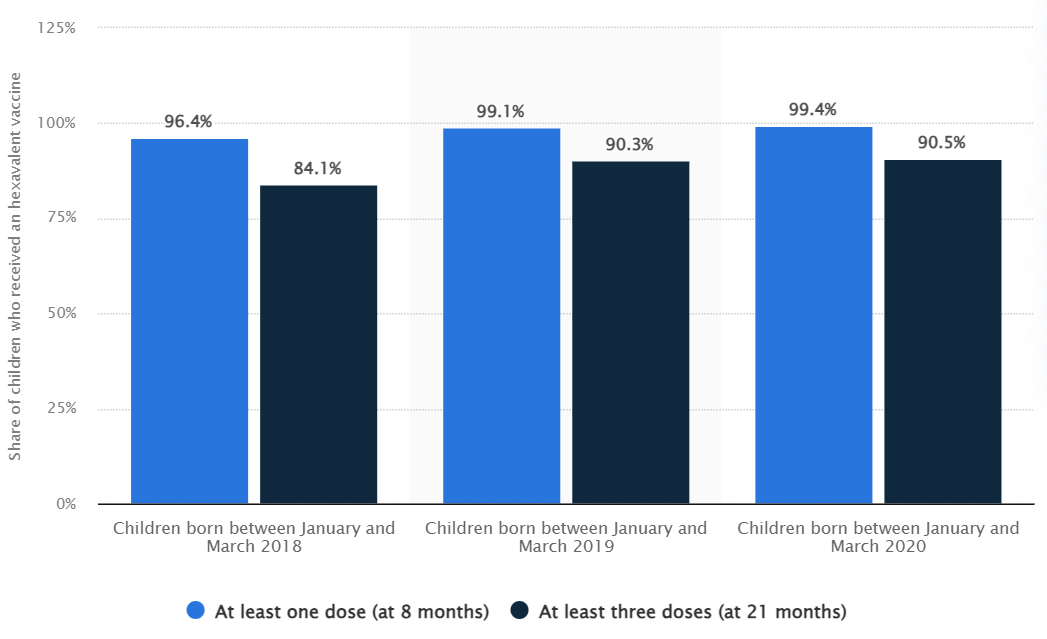
Data source: https://www.statista.com/statistics/1318258/hexavalent-vaccine-coverage-against-dtp-hib-and-hepb-in-france/
Case country 3: Germany
Germany’s immunization program includes four doses of hexavalent vaccine (DTaP-Hib-HepB-IPV), which are given to infants and young children at the age of 2, 4, and 11 months. Tdap is given as a booster dose at the age of 5 years. In addition, Germany has also included varicella vaccine in its immunization program, and MMR and varicella monovalent vaccines are recommended to be given to infants and young children at 11 months of age, while MMRV quadrivalent vaccine is recommended to be given to infants and young children at 15 months of age as the second dose because of the high response rate.
Selected vaccines and immunization schedule
| Type of vaccine | Vaccination Schedule | Note | NO. of doses | ||||
| 2 months | 4 months | 11 months | 15 months | 5-6 years old | 6 | ||
| DTaP-Hib-HepB-IPV | first dose | second dose | third dose | ||||
| Tdap | first dose | Booster shots between 8 years of age | |||||
| MMR* | first dose | MMR and monovalent varicella vaccine is preferably administered as two separate injections due to higher rates of febrile seizures following immunization with MMRV, MMRV is preferably administered as the second dose. | |||||
| MMRV | first dose | ||||||
| VAR | first dose* | ||||||
Data source: https://immunizationdata.who.int/global?topic=Vaccination-schedule&location=DEU
*The first MMR dose can be administered from 9 months of age in specific circumstances. In this case, the second MMR dose should be given as early as possible in the second year of life. Catch-up is recommended up until and including 17 years of age.
A recent study found that the vaccination rate for the hexavalent vaccine among eligible children in Germany is relatively high. Among 329 German children with inflammatory bowel disease (IBD) and autoimmune hepatitis (AIH), the full vaccination rate (four doses) for the hexavalent vaccine reached 89%, and the full vaccination rate (two doses) for the MMRV combination vaccine was 92% 7.
Case country 4: United States
According to the WHO immunization dashboard data, the U.S. immunization program includes hexavalent vaccine (DTaP-Hib-HepB-IPV) with the first, second, and third doses scheduled for infants at the ages of 2, 4, and 6 months, respectively. The MMRV quadrivalent vaccine is administered in two doses regimen at one and four years of age. The types of vaccines and vaccination schedules are shown in the table below:
Selected vaccines and immunization schedule
| Types of Vaccines | Vaccination Schedule | No. of Dose | |||||
| 2 months | 4 months | 6 months | 12 months | 15 months | 4 years | 5-7 (depends on different vaccines) | |
| DTaP-Hib-HepB-IPV | First dose | Second dose | Third dose | ||||
| DTaP-Hib-IPV | First dose | Second dose | Third dose | Forth dose | |||
| DTaP-HepB-IPV | First dose | Second dose | Third dose | ||||
| MMRV | First dose | Second dose | |||||
| Varicella | First dose | Second dose | |||||
Data source: https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-country_name?DISEASECODE=&TARGETPOP_GENERAL=
Vaccines included in the U.S. Vaccines for Children (VFC) program
In the United States, eligible children can receive free government-funded vaccines through the Vaccines for Children (VFC) program, which includes not only the traditional monovalent and low-cost vaccines, but also nearly all of the quadrivalent, pentavalent, and hexavalent vaccines available in the market. The use of higher-valent combination vaccines can be determined by consulting the pediatrician during the vaccination visit.
| Type of vaccine | Product Name |
| DTaP | Daptacel® Infanrix® |
| Hib (PRP-T) Hib (PRP-OMP) | ActHIB® Hiberix® PedvaxHIB® |
| HepB | Engerix-B® Recombivax HB® |
| MMR | M-M-R II® Priorix® |
| IPV | IPOL® |
| Tdap | Adacel® Boostrix® |
| VAR | Varivax® |
| DTaP-IPV-HepB | Pediarix® |
| DTaP-IPV-Hib | Pentacel® |
| DTaP-IPV | Kinrix® Quadracel® |
| DTaP-IPV-Hib-HepB | Vaxelis® |
| MMRV | Proquad® |
Data source: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html
According to the U.S. CDC, between 2018 and 2019, the full vaccination (four doses) rate for DTaP-containing vaccine was 81.9%, the three-dose coverage for polio was 93.4%, the first-dose coverage for MMR was 91.4%, and the first-dose coverage for varicella vaccine was 91.1%. The CDC noted that the data for the MMR and varicella vaccines may have included the MMRV quadrivalent vaccine’s coverage.
Case country 5: Cambodia
Cambodia provides free pentavalent vaccine(DTwP-Hib-HepB) for children with the support of the Global Alliance for Vaccine Immunization (Gavi). Unlike the developed countries, Cambodia does not include mumps vaccine in its current immunization program and still uses oral polio vaccine.
Selected vaccines and immunization schedule
| Type of vaccine | Vaccination Schedule | No. of doses | |||||
| At birth | 1 month | 2 months | 3 months | 9 months | 18 months | 10 | |
| DTwP-Hib-HepB | first dose | second dose | third dose | ||||
| HepB (Monovalent) | first dose | ||||||
| IPV | first dose | ||||||
| OPV | first dose | second dose | third dose | ||||
| MR | first dose | second dose | |||||
Data source: https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-country_name?DISEASECODE=DIPHTHERIA&TARGETPOP_GENERAL=
A study based on community data published in 2023 shows that among Cambodian children born between 2012 and 2018, the first dose of pentavalent vaccine (DTwP-Hib-HepB) had a high coverage rate of 96%, and the full course (three doses) coverage rate was 73% 9. According to Cambodia Demographic and Health Survey (DHS) 2021-2022 data, full vaccination (three doses) coverage of DTwP-Hib-HepB vaccine was 83.7% among males, 84.5% among females, and overall full vaccination coverage was 84.1% 10.
Case country 6: Malaysia
Malaysia, a pioneer in introducing high-valent pediatric combination vaccines as non-Gavi-eligible middle-income countries. As early as 20026, Malaysia introduced DTwP-Hib-HepB pentavalent vaccine in its NIP and transited it to DTaP-Hib-HepB pentavalent vaccine in 2008. In 2020, they introduced the hexavalent vaccine.
Selected vaccines and immunization schedule
| Type of vaccine | Vaccination Schedule | Note | No. of doses | ||||||
| At birth | 2 months | 3 months | 5 months | 9 months | 12 months | 18 months | 7 | ||
| DTaP-Hib-HepB-IPV | first dose | second dose | third dose | fourth dose | |||||
| HepB | first dose | From November 2020, only the hepatitis b birth dose will be available. Subsequent doses are offered by hexavalent vaccine. | |||||||
| MR | first dose | second dose | |||||||
Data source: https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-country_name?DISEASECODE=&TARGETPOP_GENERAL=
According to the National Health and Morbidity Survey of Malaysia (NHMS), the first dose coverage of Pentavalent vaccine in 2016 reached 89.8%, and the full vaccination (three doses) rate was 89.0% 11.
Case country 7: Japan
Japan has been relatively conservative in using the pediatric combination vaccines. A series of adverse events after the MMR vaccination in 1989 makes Japanese policymakers be cautious about vaccine-related risks. It was not until 2012 that Japan began using conjugate vaccines containing polio antigens. The Japanese government favors products with excellent safety records when selecting vaccines.
Selected vaccines and immunization schedule
| Type of vaccine | Vaccination Schedule | No. of doses | ||||||||
| 2 months | 3 months | 4 months | 6 months | 7 months | 12 months | 13 month | 18 months | 5 years old | 14 | |
| DTaP-IPV | first dose | second dose | third dose | |||||||
| HepB | first dose | second dose | third dose | |||||||
| Hib | first dose | second dose | third dose | fourth dose | ||||||
| MR | first dose | second dose | ||||||||
| VAR | first dose | second dose | ||||||||
Data source: https://immunizationdata.who.int/pages/schedule-by-country/jpn.html?DISEASECODE=&TARGETPOP_GENERAL=
According to the statistics from the Ministry of Health, Labor and Welfare in Japan in 2020, the coverage rate for the first dose of the measles-rubella (MR) bivalent vaccine was 98.5%, and the full-dose coverage rate was 94.7%. For the DTaP-IPV quadrivalent vaccine, the first dose coverage was 101.3%, and the full vaccination rate was 105.5% (the target population refers to the population that newly meets the immunization criteria each year, while the implementation population refers to the total number of people who receive immunization among those who are eligible each year. Hence, the implementation rate can exceed 100%) 12.
Case country 8: Indonesia
Indonesia introduced the pentavalent vaccine (DTwP-Hib-HepB) into its National Immunization Program in 201313. According to data from the World Health Organization (WHO), the pentavalent vaccine in Indonesia applies 4-dose schedule administered at 2, 3, 4, and 18 months of age. The hepatitis B vaccine (HepB) requires only a single dose at birth. The measles-rubella (MR) bivalent vaccine is given by a two-dose regimen, administered at 9 and 18 months of age. The inactivated polio vaccine (IPV) is given at 4 and 9 months. The oral polio vaccine (OPV) is given in a total of four doses, consecutively at birth, 1 month, 2 months, and 3 months of age.
Selected vaccines and immunization schedule
| Type of vaccine | Vaccination Schedule | total number of doses | ||||||
| birth | 1 month | 2 months | 3 months | 4 months | 9 months | 18 months | 6 | |
| DTwP-Hib-HepB | first dose | second dose | third dose | fourth dose | ||||
| HepB | first dose | |||||||
| MR | first dose | second dose | ||||||
| IPV | first dose | second dose | ||||||
| OPV* | first dose | second dose | third dose | fourth dose | ||||
Data source: https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-country_name?DISEASECODE=&TARGETPOP_GENERAL=
*To optimize protection against polio, a second dose of IPV or IPV2 has been added to the routine immunization schedule since 2022. This combination of 4 doses of bOPV and 2 doses of IPV has been recommended by WHO and the Indonesian Technical Advisory Group on Immunization (ITAGI)
According to the World Health Organization’s (WHO) report on immunization coverage in 2023, Indonesia achieved an 85% coverage for the DTwP-Hib-HepB pentavalent vaccine in 202314.
Content Editor: Menglu Jiang
Page Editor: Ziqi Liu
Reference
1 Parthasarathy, A. Safety of pentavalent vaccines. Indian Pediatrics. 2013 December; 50(12): 1162.
2. Khan MM, Vargas-Zambrano JC, Coudeville L. How did the adoption of wP-pentavalent affect the global paediatric vaccine coverage rate? A multicountry panel data analysis. BMJ Open. 2022 Apr 4;12(4):e053236. doi: 10.1136/bmjopen-2021-053236.
3 崔富强. 中国儿童用联合疫苗免疫策略的探讨.中国病毒病杂志.2019年第9期.6.
4 GAVI. Pentavalent vaccine support. https://www.Gavi.org/types-support/vaccine-support/pentavalent#:~:text=Gavi%20began%20supporting%20pentavalent%20vaccine,part%20of%20the%20pentavalent%20vaccine. Accessed Sep. 15, 2024.
5 NHS. Childhood Vaccination Coverage Statistics – England, 2021-22. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisation-statistics/2021-22#:~:text=Vaccine%20coverage%20in%202021%2D22%20decreased%20compared%20to%202020%2D21.&text=Coverage%20for%20the%205%2Din1,94.4%25%20in%202021%2D22.&text=MMR1%20coverage%20at%2024%20months,from%2094.3%25%20the%20previous%20year. Accessed Sep. 15, 2024.
6 Statista. Hexavalent vaccination coverage against Diphtheria, Tetanus, Poliomyelitis, Pertussis, Hemophilus influenzae type B, and Hepatitis B in France, among children born between 2018 and 2020. https://www.statista.com/statistics/1318258/hexavalent-vaccine-coverage-against-dtp-hib-and-hepb-in-france/. Accessed May 08, 2023.
7 Cagol, L., Seitel, T., Ehrenberg, S., Frivolt, K., Krahl, A., Lainka, E., Gerner, P., Lenhartz, H., Vermehren, J., Radke, M., Trenkel, S., Mayer, B., Koletzko, S., Debatin, K. M., Mertens, T., & Posovszky, C. (2020). Vaccination rate and immunity of children and adolescents with inflammatory bowel disease or autoimmune hepatitis in Germany. Vaccine, 38(7), 1810–1817. https://doi.org/10.1016/j.vaccine.2019.12.024
8 U.S. CDC. Vaccination coverage by age 24 months among children born during 2018-2019 – National Immunization Survey – Child, United States, 2019-2021. https://www.cdc.gov/mmwr/volumes/72/wr/mm7202a3.htm#T1_down. Accessed May 20, 2023.
9 Verrier, F., de Lauzanne, A., Diouf, J. N., Zo, A. Z., Ramblière, L., Herindrainy, P., Sarr, F. D., Sok, T., Vray, M., Collard, J. M., Borand, L., Kermorvant-Duchemin, E., Delarocque-Astagneau, E., Guillemot, D., Huynh, B. T., & Bacterial Infections and Antibiotic-Resistant Diseases Among Young Children in Low-Income Countries (BIRDY) Study Group (2023). Vaccination Coverage and Risk Factors Associated with Incomplete Vaccination Among Children in Cambodia, Madagascar, and Senegal. Open forum infectious diseases, 10(4), ofad136. https://doi.org/10.1093/ofid/ofad136
10 National Institute of Statistics (NIS) [Cambodia], Ministry of Health (MoH) [Cambodia], and ICF. 2023. Cambodia Demographic and Health Survey 2021–22 Final Report. Phnom Penh, Cambodia, and Rockville, Maryland, USA: NIS, MoH, and ICF.
11 Lim, K. K., Chan, Y. Y., Noor Ani, A., Rohani, J., Siti Norfadhilah, Z. A., & Santhi, M. R. (2017). Complete immunization coverage and its determinants among children in Malaysia: findings from the National Health and Morbidity Survey (NHMS) 2016. Public health, 153, 52–57. https://doi.org/10.1016/j.puhe.2017.08.001
12 日本厚生劳动省2020年数据:https://www.mhlw.go.jp/topics/bcg/other/5.html
13. Hadisoemarto, P. F., Reich, M. R., & Castro, M. C. (2016). Introduction of pentavalent vaccine in Indonesia: a policy analysis. Health policy and planning, 31(8), 1079–1088. https://doi.org/10.1093/heapol/czw038
14. Indonesia: WHO and UNICEF estimates of immunization coverage: 2023 revision. https://www.who.int/publications/m/item/immunization-2024-indonesia-country-profile





