By Article 12 of Chapter 3 of the Rules for Vaccine Manufacturing and Distribution, a marketing authorization holder (MAH) may apply for entrusted manufacturing under any of the following circumstances:
- The industry and information technology administration of the State Council proposes reserve needs and believes that the MAHs existing manufacturing capacity cannot meet the needs.
- The health administration of the State Council proposes an urgent need for disease prevention and control and believes that the MAH’s existing manufacturing capacity cannot meet the needs.
- Polyvalent and multivalent vaccines are to be produced.
In the public consultation for the Rules’ first draft, the National Medical Products Administration (NMPA) did not include the term 3 – the production of polyvalent and multivalent vaccines – for entrusted manufacturing. In the second draft of the Rules, the NMPA added term 3 but required both the commissioning party and the commissioned party to be legitimate pharmaceutical manufacturers. In addition, both parties need to have a shareholding relationship, meaning one party must hold more than 50% of the other party’s shares or both are subsidiaries of the same pharmaceutical entity with more than 50% share for the subsidiaries. Later, when the NMPA officially released the Rules, the requirements for entrusted manufacturing parties in term 3 were deleted, indicating a more open and inclusive attitude towards joint R&D and entrusted manufacturing for combination vaccines.
It is worth noting that there are two requirements for entrusted vaccine manufacturing. First, the scope of the entrusted manufacturing should cover the whole process of vaccine production. With special approval from the NMPA, the entrusted manufacturing could be limited to producing the bulk of the preparation. Second, the commissioned contract shall not be transferred to a third party for entrusted manufacturing.
The application process and required materials for entrusted manufacturing:
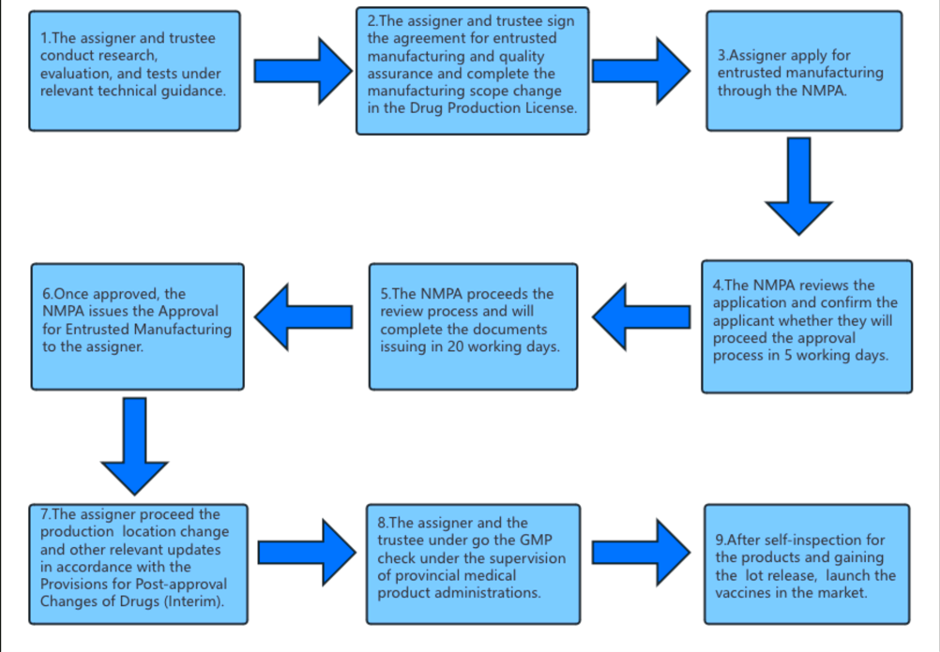
The materials for contract manufacturing applications (MAHs) include:
- Vaccine Entrusted Manufacturing Application Form
- Commissioned Agreement and Quality Assurance Agreement
- The general statement for entrusted manufacturing, including reasons for application, scope, and time limitation
- Evaluation report for the commissioned party by the commissioning party
- Other supporting documents by the Rules
Content Editor: Menglu Jiang
Page Editor: Ziqi Liu





