Policy Updates
01
11 provinces in China have launched free HPV vaccination at the provincial level
Since 2022, 11 provinces(including autonomous regions and municipalities directly under the central government) have implemented or launched free HPV vaccination programs for school-age girls.
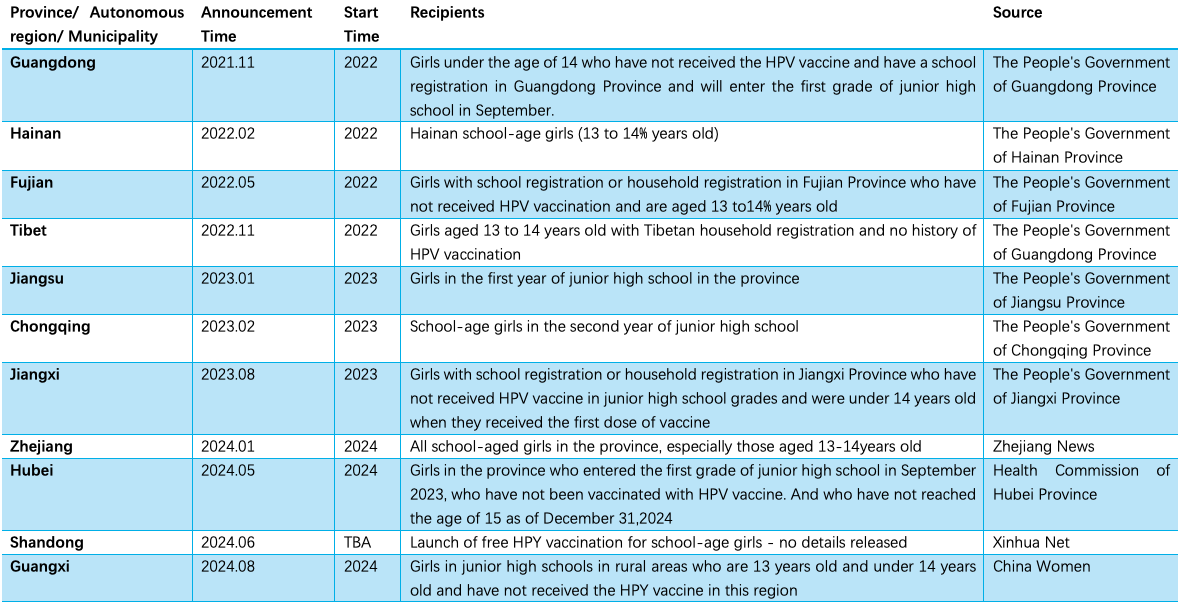
Source: Chinese Vaccinology Course (CNVAC) Wechat official account
Relative content:Regional Strategies, Policies and Practices of HPV-Vaccination: Key Issues and Challenges
Journal Content Recommendation
02
Acceptance and willingness to pay for DTaP-HBV-IPV-Hib hexavalent vaccine among parents: A cross-sectional survey in China
This study was published in Human Vaccines & Immunotherapeutics. DTaP-HBV-IPV-Hib hexavalent vaccine has been used in high-income countries for many years to prevent diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, and invasive Haemophilus influenzae type b disease. Currently, no hexavalent vaccines have been approved for use in China. The aim of this study was to investigate the parental acceptance and willingness-to-pay (WTP) for a hexavalent vaccine in a cross-sectional survey conducted in 16 vaccination clinics in 7 cities in China.
Between April 28 and June 30, 2023, a total of 581 parents of children aged 0–6 years participated in the survey; 435 (74.87%, 95% CI:71.3%−78.4%) parents indicated acceptance of hexavalent vaccine. Residence location, parents’ education level, experience paying for vaccination, and disease knowledge scores were key factors affecting parents’ choices for vaccination. Mean (SD) and median (IQR) willingness to pay for full 4-dose course vaccination were 2266.66 (SD=1177.1) CNY and 2400 (IQR: 1600–2800) CNY.Children’s age (p < 0.001), parents’ education level (p = 0 .024), and perceived price barriers (p < 0.001) were significantly associated with WTP.
The study showed that parental acceptance and willingness-to-pay for the hexavalent vaccine was high. The less money parents have to pay out of pocket, the more willing they would be to accept the vaccine. Therefore, the acceptance level may increase if the vaccine is covered by medical insurance, provided free , or if its price is reduced. The results of the study provide reference for optimizing and adjusting immunization strategies in China.
https://doi.org/10.1080/21645515.2024.2333098
03
HPV vaccine behaviors and intentions among a diverse sample of women aged 27-45 years: implications for shared clinical decision-making
This study, published in BMC Public Health, examined the impact of The Advisory Committee on Immunization Practices (ACIP) recommendations for shared clinical decision-making (SCDM) for HPV vaccination in persons aged 27 to 45 years, which involves adequate communication and shared decision making between doctors and patients regarding the pros and cons of HPV vaccination. The study aimed to understand the HPV vaccination behaviors of women in this age group and its influencing factors, to inform the promotion of vaccination rates. The study conducted a cross-sectional online survey among 324 U.S. women aged 27-45, recruited through a Qualtrics™ respondent panel.
Results showed that only 31.1% had at least one dose of the HPV vaccine. In multivariable analyses,younger women aged 27-29 years were more likely to be vaccinated (adjusted odds ratio (AOR) = 0.50; 95% CI: 0.26-0.96) and showed a stronger belief in the effectiveness of the HPV vaccine (AOR=2.75; 95% CI: 1.33-5.71). Of those unvaccinated or unsure, 54.8% indicated they were likely to get vaccinated in the future. In particular, women with abnormal past cervical cancer screening results or a positive HPV test were more inclined to receive the HPV vaccine.Compared with those who did not intend to be vaccinated, those reporting a belief that vaccines are well tested (AOR = 2.28; 95% CI: 1.12–4.63), that they were likely to get an HPV infection (AOR = 2.66; 95%CI = 1.16–6.05), and that they were comfortable asking a health care provider for the HPV vaccine (AOR = 4.53; 95%CI: 1.59–12.88) had greater odds of intending to be vaccinated. In contrast, those reporting that previous negative healthcare experiences would influence their HPV vaccination decision had lower odds of intending to be vaccinated (AOR = 0.40; 95% CI: 0.20–0.80) .
The study concluded that efforts to promote informed decision-making among mid-adult women may include educating women about the rigorous vaccine testing and approval process, their risk factors for HPV infection, and encouraging them to engage in SCDM with their medical providers. In addition, targeted to reach women who have had negative experiences with healthcare may also be needed.
https://doi.org/10.1186/s12889-024-18740-2
04
Long-term effect of pneumococcal conjugate vaccines on invasive pneumococcal disease incidence among people of all ages from national, active, laboratory-based surveillance in South Africa, 2005–19: a cohort observational study
This study was published in The Lancet Global Health. In South Africa, 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in 2009 and 13-valent PCV (PCV13) was introduced in 2011, both in a 2+1schedule. The study evaluated the long-term preventive effect of PCV on invasive pneumococcal disease (IPD) in different age groups based on national active laboratory surveillance data from 2005 to 2019.
A total of 52 957 IPD cases identified in the surveillance, of which 50,705 provided age data, including 9,398 (18.5%) infants aged younger than 2 years. Compared with expected case numbers (no vaccination) predicted using all available data, overall IPD rates among children younger than 2 years declined by 76.0% (percentage risk difference; 95% CI –79.0 to –72.8%) in 2019; notably, PCV7 and additional PCV13 serotype IPD rates declined by 95.5% (–97.0 to –93.4) and 93.8% (–96.2 to –90.5), respectively, whereas non-vaccine serotypes (NVTs) did not change significantly. Among adults aged 25–44 years, overall IPD declined by 50·4% (–54.2 to –46.3%), and PCV7 and additional PCV13 serotype IPD rates declined by 86·1% (–88.7 to –83.1%) and 77.2% (–80.9 to –73.0%), respectively, whereas NVTs increased by 78.5% (56.8 to 103.4%). Individuals aged older than 64 years also benefited from declines in IPD (–30.2%; –41.9 to –16.2%), but NVTs increased (234.9%; 138.1 to 379.4%).
The study demonstrated that PCV consistently provided long-term direct and indirect protective benefits across all age groups, with NVT increases in adults older than 24 years.
https://doi.org/10.1016/S2214-109X(24)00263-8
05
Antibody persistence to diphtheria toxoid, tetanus toxoid, Bordetella pertussis antigens, and Haemophilus influenzae type b following primary and first booster with pentavalent versus hexavalent vaccines
This study was published in Human Vaccines & Immunotherapeutics. Thailand has incorporated the whole-cell (wP) pertussis vaccine into the expanded program on immunization since 1977 and has offered the acellular pertussis (aP) vaccine as an optional vaccine for infants since 2001. The study followed healthy children from a clinical trial in which children were randomly assigned to receive either pentavalent (DTwP-HB-Hib) or hexavalent (DTaP-IPV-HB-Hib) vaccines for their primary series (administered at 2, 4, and 6 months) and first booster vaccination (18 months). Both groups received Tdap-IPV as a second booster at the age of 4 years. The study evaluated antibody persistence to diphtheria toxoid (DT), tetanus toxoid (TT), and Bordetella pertussis (B. pertussis) between 2 and 6 years of age by the annual collection of blood samples, and for the immunogenicity study of Tdap-IPV at 1 month after the second booster. Antibody persistence to Haemophilus influenzae type b (Hib) was also followed until 3 years old.
The results of the study showed that a total of 105 hexavalent-vaccinated children and 91 pentavalent-vaccinated children completed this study. Both pentavalent and hexavalent groups demonstrated increased antibody levels against DT, TT, and B. pertussis antigens following the second booster with Tdap-IPV. All children achieved a seroprotective concentration for anti-DT and anti-TT IgG at 1 month post booster. The hexavalent group possessed significantly higher anti-pertactin IgG (adjusted p = 0.023), whereas the pentavalent group possessed significantly higher anti-pertussis toxin IgG (adjusted p < 0.001) after the second booster. Despite declining levels post-second booster, a greater number of children sustained protective levels of anti-DT and anti-TT IgG compared to those after the first booster.
In conclusion, the Tdap-IPV vaccine induced a robust antibody response in four-year-old children, with all children achieving seroprotective concentrations for anti-DT and anti-TT IgG at one-month post-booster. Further analysis revealed that anti-PT IgG levels were significantly higher in the wP-vaccinated than in the aP-vaccinated cohorts after the Tdap-IPV booster. This study contributes valuable insights into how different vaccination regimens impact the immune response to childhood vaccination.
https://www.tandfonline.com/doi/full/10.1080/21645515.2024.2352909
Content Editor: Ziqi Liu
Page Editor:Ziqi Liu





