A recent study published in Human Vaccines & Immunotherapeutic analyzed the key decision factors for introducing HPV vaccination programs in low- and middle-income countries (LMICs). The study included Asian and African countries that have already introduced or are planning to introduce HPV vaccinations at the national level.
The researchers identified potential stakeholders and invited them to participate in semi-structured interviews. Of the 31 interviewees who accepted the invitation, 18 were stakeholders at the national level, mainly from the national immunization planning departments and non-profit organizations. The other 13 were stakeholders at the global level, mainly from collaborative research institutions, intergovernmental organizations for global health, and non-profit organizations.
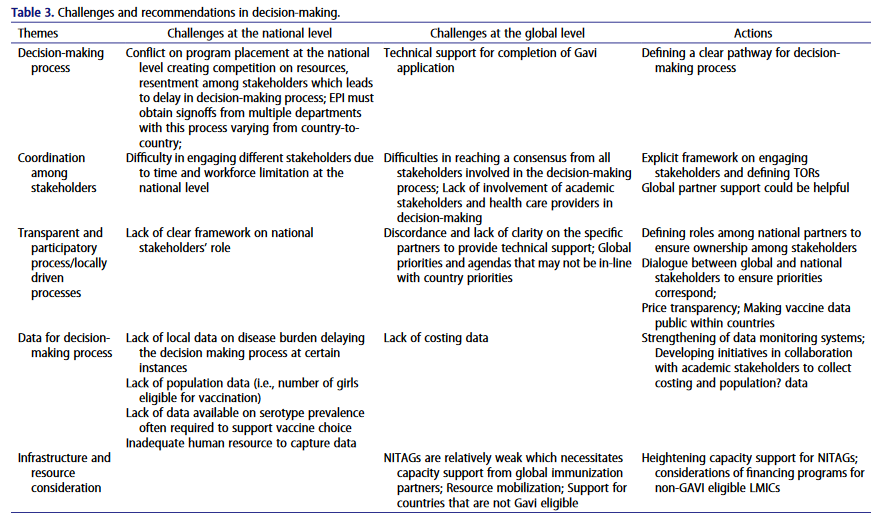
Findings showed that the decision-making process for HPV vaccination requires the participation of multiple national and global institutions and stakeholders and that the process varies from one country to another. Conjunctively, most countries establish National Immunization Technical Advisory Groups (NITAGs). Global immunization partners such as WHO, UNICEF, or Gavi may present evidence to NITAGs and discuss options and next steps for applying for the HPV vaccine.
Once a decision is made to continue introducing the HPV vaccine, the national immunization program team starts discussions with technical advisory groups such as NITAG. NITAG will independently assess factors such as the country’s disease burden, national capacity and resources, and health financing. The introduction of a specific vaccine is usually discussed by a core expert group. After receiving the advice from NITAG, the Ministry of Health, the national immunization program team, and relevant stakeholders cooperate in preparing a report and submitting it to the Inter-Agency Coordination Committee (ICC) for review. After the ICC makes recommendations and responses, the national immunization program team starts the Gavi vaccine application process. The interview outcomes showed that in many cases the national immunization program team did not have the decision-making power and that the process involved decision-making by multiple departments, which varied from country to country.

The research concluded four key factors in the decision-making process, including considerations of partnerships and project coordination, locally driven vaccine introduction decisions, high-quality data availability, and infrastructure and resource situation.
In consideration for partnerships and project coordination, stakeholders at the national and global levels play important roles in vaccine decision-making. Interviews showed that stakeholders involved in national-level decision-making include the Ministry of Education, the Ministry of Finance, professional associations, and development partners/global partners. Multiple departments within the Ministry of Health, such as the Maternal and Child Health Department, Women’s Affairs Department, Youth and Reproductive Health Service Department, Community Health Department, and Social Affairs Department are also involved. Global-level stakeholders are involved in the entire decision-making process and play a variety of roles, including financing, data collection, providing relevant data, advocacy, communication, and capacity building.
Vaccine introduction decisions are largely led by countries. Vaccine decision-making is a process substantially driven by countries themselves, but external influence from global organizations has played a key role in shaping a country’s decision-making process. In most cases, high disease burden, high mortality rates, availability of funds, and advocacy from local stakeholders are the primary drivers for HPV vaccine prioritization in immunization plans. At the same time, demands and pressures from the World Health Organization, as well as transparency of information from global and national stakeholders involved in vaccine decision-making can also influence decisions, with higher international pressures and higher levels of message transparency often facilitating the advancement of vaccine decision-making.
Regarding the availability of high-quality data, national and global stakeholders have reported the importance of having local data and generating local evidence through pilot/demonstration projects, with an emphasis on countries conducting their own assessments and leading data collection processes. Having local data and establishing local databases is advantageous to the decision-making process of all global and national stakeholders. However, local data from most countries are either not available or not being used, thus delaying the decision-making process.
At the global level, the decision-making process involves considering several factors related to infrastructure and resources, including health system capacity, the role of technical advisory groups, and financing and budget allocations. Because lower- and middle-income countries not only need vaccines but also appropriate guidelines, effective promotion and dissemination methods, and sustainable financing to implement vaccination programs.
The introduction of the HPV vaccine has been more successful compared to the introduction of traditional childhood vaccines. In fact, the selection of an effective delivery platform to promote HPV vaccine coverage among adolescent girls remains a challenge in the implementation of HPV vaccination programs. Many low and middle-income countries lack attention to adolescent health, resulting in poor development of adolescent health services, which poses a considerable challenge to the implementation of HPV vaccination programs.
Finally, the study concludes that while achieving the WHO goal of eliminating cervical cancer has become a global priority, future decision-making should focus on communication and coordination among different stakeholders, ensure locally led decision-making processes, increase the availability of high-quality data for decision-making, and provide the necessary resources for countries to drive decision-making.
Read More: https://doi.org/10.1080/21645515.2022.2150454
The original article “Key decision-making factors for human papillomavirus (HPV) vaccine program introduction in low-and-middle-income-countries: Global and national stakeholder perspectives” was published in Human Vaccines & Immunotherapeutics on December 9,2022.





