Streptococcus pneumoniae (Spn) is a major pathogen causing serious diseases such as pneumonia, meningitis, and bacteremia among children. Infections caused by Spn are common in infants and young children, the elderly, and people with underlying diseases. Resistance related to streptococcus pneumoniae is a growing concern due to the widespread use of antibiotics in clinical treatment, and this further increases the burden of disease and the difficulty of public health management. China is one of 41 countries worldwide that has not yet included pneumococcal conjugate vaccine (PCV) in its national immunization programs. A random-effects meta-analysis model estimated a level of 21.7% for PCV vaccine coverage in China in 2021, which is lower than the coverage rate in high-income countries and even those in some low- and middle-income countries during the same period.
A systematic review published in Human Vaccines & Immunotherapeutics shows that the introduction of the 10-valent pneumococcal conjugate vaccine in Brazil for 10 years has made positive impacts based on different studies – including a reduction in deaths and hospitalizations related to invasive pneumococcal disease (IPD) in children under five years of age, and a reduction in the disease burden of acute otitis media and nasopharyngeal pneumococcal carriage.
In 2010, the 10-valent pneumococcal conjugate vaccine (PHiD-CV, non-separable Haemophilus influenzae protein D as the carrier) was introduced in the routine national immunization program for Brazilian children. The initial immunization schedule was 3+1 (vaccination at 2, 4, and 6 months of age, with a booster at 12-15 months of age), which was later changed in 2016 to be a 2+1 immunization schedule (vaccination at 2 and 4 months of age, with a booster at 12 months of age), as well as a 1-dose catch-up program for children aged 1-4 years who were never vaccinated previously.
A total of nineteen peer-reviewed articles were included in the study as well as ten conference abstracts. Among them, nine studies reported the national-level data in Brazil and the remaining studies provided findings at the state or regional level, involving participants at different ages including children, adolescents, and the whole population. Ten of the abovementioned studies reported information related to invasive pneumococcal disease (IPD), nine studies involved all-cause pneumonia, and three studies mentioned community-acquired pneumonia. In addition, evidence related to pneumococcal meningitis, acute otitis media, and nasopharyngeal pneumococcal carriage were also included in the literature.
According to the data from Brazilian Ministry of Health, vaccine coverage rate is calculated based on the completion of the last dose of basic immunization. The data show that the vaccination rate of the basic immunization sequence in Brazil exceeded 80% in 2011-2019 (in 2010-2015 the basic immunization sequence was the completion of three doses at 2, 4 and 6 months of age, and after 2016 the completion of two doses at 2 and 4 months of age), and the booster vaccination rate exceeded 70% between 2013 and 2019. Different studies have shown that the direct and indirect disease burden of invasive pneumococcal disease (IPD) as well as pneumococcal meningitis in children under five years of age has decreased after the introduction of PHiD-CV vaccine in Brazil. For example, one study showed that the introduction of PhiD-CV resulted in a 44.4% reduction in the incidence of IPD and pneumococcal meningitis among children aged 2 months to <2 years nationwide between 2008 and 2013.
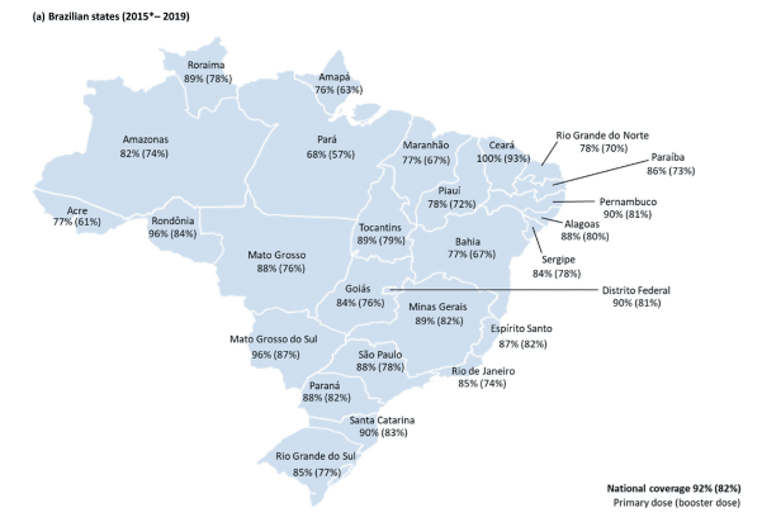
In addition, evidence from different studies shows that the introduction of the PHiD-CV vaccine reduced the pneumonia-related deaths in children under five years of age by 10%-28% and the pneumonia-related hospitalizations by 9%-26.5% nationwide and statewide in Brazil. Hospitalization rates due to pneumonia at the national level and community-acquired pneumonia (CAP) also decreased. For example, in an ecological study in Santa Catarina that analyzed data from the Mortality Information System for children under 1 year of age, an 11% reduction in all-cause pneumonia-related deaths was reported. The inclusion of the PhiD-CV vaccine resulted in 43%-84% protection against acute otitis media among children under 23 months of age.
The review also assessed the prevalence of nasopharyngeal Streptococcus pneumoniae carriage in infants and children after the introduction of the vaccine. In multiple cross-sectional studies in children aged 1-2 years in the state of São Paulo, Streptococcus pneumoniae carriage increased from 40.3% in 2010 to 59.7% in 2017, with a significant decrease of 90.9% and 95.5% in vaccine-type isolates in 2013 and 2017, respectively.
Published literature also shows that after the introduction of the PHiD-CV vaccine for seven years in Brazil, the serotype distribution of Streptococcus pneumoniae changed, with a gradual increase in non-vaccine serotypes of Streptococcus pneumoniae that became pathogenic in children and the elderly.
It is also of interest to note that the vaccination rate for PHiD-CV has decreased in Brazil since 2016. At the same time, vaccination rates for other vaccines have declined too. PHiD-CV vaccine coverage was maintained at levels above 95% in the first decade of the 21st century but started to decline since 2014. Low vaccination rates may affect vaccination programs, independent with the type of PCV vaccine introduced. The reasons for the decline in vaccine coverage are not yet clear for the Brazilian Ministry of Health, and recent literature has analyzed many factors that may be relevant, such as the restructuring of the routine national immunization program for children, misconceptions about the importance of vaccines and vaccination procedures, and vaccine hesitancy.
Read More





