A recent study published in Infectious Diseases and Therapy showed that, based on time series analysis, the inclusion of rotavirus vaccine in Argentina’s National Immunization Program had a positive impact on reducing the burden of related diseases.
According to statistics from the National Healthcare Surveillance System (SNVS, Sistema Nacional de Vigilancia de la Salud) of Argentina, two years after the rotavirus vaccine was included in the national immunization plan (2017-2018), the number of acute diarrheal cases, rotavirus cases, and the monthly hospital discharge rate significantly decreased among children under two years of age. This decrease was accompanied by the weakening of the seasonal rotavirus epidemic trend and shortening of the epidemic’s duration.
In January 2015, Argentina included the rotavirus vaccine (Rotarix, GSK, Belgium) in its national immunization program. The monovalent vaccine, made from the human strain G1P, is administered orally in a two-dose regimen for infants aged two and four months. In the first year of introduction, rotavirus vaccine coverage reached 61% (note: baseline vaccination coverage data is not available prior to 2015). Vaccine coverage in 2016, 2017 and 2018 was 75%, 78% and 80%, respectively. According to estimates from the World Health Organization, the rotavirus vaccine rate in Argentina in 2021 is expected to increase by 74% compared with 2015.
Acute diarrheal disease (ADD) in children is a notifiable infectious disease in Argentina. The National Medical Healthcare Monitoring System of Argentina collects and reports the data regarding the current status of rotavirus disease countrywide. This study used all the medical consultation records of acute diarrhea in children from the clinical monitoring units, as well as analyzed a number of laboratory-tested cases and their causes reported by the laboratory monitoring units. The hospital discharge data were provided by the Office of Health Statistics and Information (DEIS, Dirección de Estadística e Información en Salud). All data were categorized into three age groups: children under 1 year old, children under 2 years old, and children between 2-5 years old. In this study, the impact of vaccination was assessed by using the data from 2012-2014 and compared with the vaccination data from 2015-2018. The purpose of this study was to evaluate the impact of vaccination on reported cases of acute diarrhea and rotavirus, as well as to the hospital discharge rates.
In this paper, the researchers defined the two-year impact of rotavirus vaccine inclusion in the national immunization program and determined four main aspects. These aspects include the number of reported cases of rotavirus, the number of children discharged from hospital with acute diarrhea, the number of reported cases of acute diarrhea, and the number of discharged patients after rotavirus infection. All of these aspects were applicable for all three age groups.
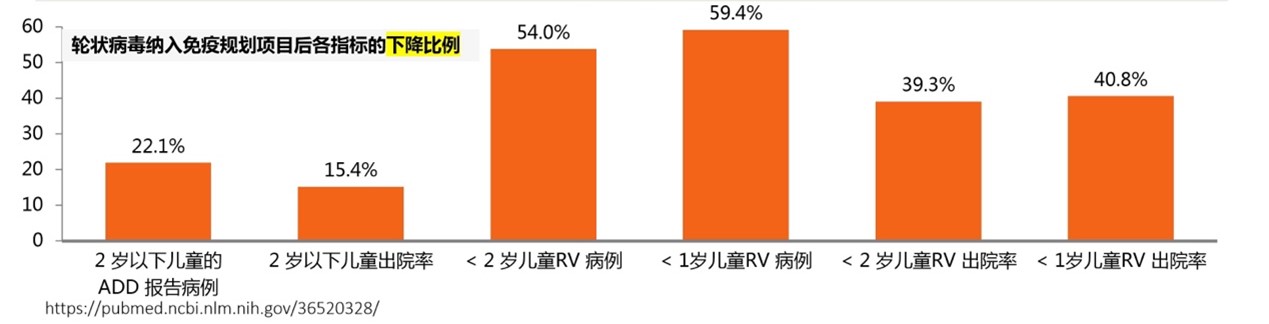
Overall, since the vaccine introduction in January 2015, a decrease in the number of cases and hospital discharges per month was observed for both ADD and RV cases. Compared with the age group of 2-5 years, the reduction in the number of cases and discharged patients in the age group under 2 years was more obvious. Rotavirus vaccine significantly reduced the number of reported cases of acute diarrhea in children, with the percentage of cases averted by vaccination varying from 12.9% to 26.3% per month. A total of 233,947 cases of acute diarrhea in children have been prevented since the vaccine introduction. The study revealed that the vaccine had a greater impact on rotavirus cases decrease than on ADD. Several years after the introduction of the rotavirus vaccine, the number of reported RV cases in all age groups decreased significantly in comparison to the ADD. In the hospital, a total of 3,323 rotavirus cases were averted in the <2 years age group.
Moreover, the number of ADD and RV hospital discharges among children decreased accordingly. In the <1 year old and <2 years olds age groups, the hospital discharges varied from 19.0% and 15.4%, respectively. In total, there were 4,263 fewer discharges among children from <2 years old age group, which indicates that these cases were potentially prevented by the vaccine. A statistically significant reduction in hospital discharges was observed for the two youngest age groups. The reduction percentage of prevented cases averaged at about 40% per month. The study also reports that a rotavirus vaccine could attenuate seasonal rotavirus prevalence patterns.
Researchers also pointed out that the number of ADD cases in Argentina is still underestimated. The main reason for this is that the infected people do not choose to seek medical treatment due to mild and self-limiting symptoms. Second, some provinces in Argentina lack complete hospital discharge records and were excluded from the data analysis. This study also paves the way for cost-benefit analysis of rotavirus vaccination and rotavirus-related diseases. It will provide an important basis for subsequent clinical trials, public-health decision making, and evaluation of the intervention’s efficiency.
Since 2006, multiple Latin American countries have introduced rotavirus vaccines in their national immunization programs. Brazil was the first country to introduce the rotavirus vaccine in 2006, followed by El Salvador, Mexico, Panama, and others. Both rotavirus and childhood acute diarrhea cases decreased among infants aged 5 years and younger in these countries, and the decline was more pronounced among children aged 2 and younger.
[1] Rotavirus Vaccination Coverage. Retrieved Feb 10th, 2023, from https://immunizationdata.who.int/pages/coverage/rota.html?CODE=ARG&ANTIGEN=&YEAR=
Read More:
https://link.springer.com/article/10.1007/s40121-022-00709-6
The original article ” Rotavirus Vaccine Impact since Its Introduction in the National Immunization Program of Argentina” was published in Infectious Diseases and Therapy on December 15, 2022.





