The economic evaluation of vaccines primarily involves analyzing the costs and benefits of vaccination to assess its efficacy in reducing disease burden, decreasing healthcare resource utilization, and mitigating disease-related economic costs. By quantifying the comprehensive public health and economic value of vaccination, it provides policymakers with scientific evidence to support the formulation and optimization of immunization strategies. Currently, both global and domestic studies have conducted economic evaluations on different types of rotavirus vaccines.
Cost-effectiveness analysis (CEA) is a method to evaluate the effects of different decisions or interventions relative to their costs in health or other domains, which is also applicable in rotavirus vaccination. Typically, the incremental cost-effectiveness ratio (ICER) is calculated, representing the cost per additional unit of effectiveness, and compared against a predetermined cost-effectiveness threshold (e.g., one or three times the per capita GDP). If the ICER falls below this threshold, the intervention is deemed cost-effective. Key factors influencing ICER outcomes include the design of the cost and effectiveness models, the selection of cost-effectiveness thresholds, data quality and sources, the time horizon of the analysis, as well as socioeconomic context and ethical considerations.
Global Studies
Studies have shown that introducing rotavirus vaccines into national immunization programs is highly cost-effective. A study involving 73 countries previously and currently eligible for Gavi support found that rotavirus vaccination had the potential to avert 80.7 million outpatient visits, 7.9 million hospitalization, and 576,567 deaths between 2018-2027. From the government perspective, the cost per DALY averted in Gavi countries is $264 (Figure 5.1). Additionally, results indicate that introducing rotavirus vaccines into immunization programs is more cost-effective in Africa and the Eastern Mediterranean regions, where cost-effectiveness could be achieved at lower cost thresholds1.
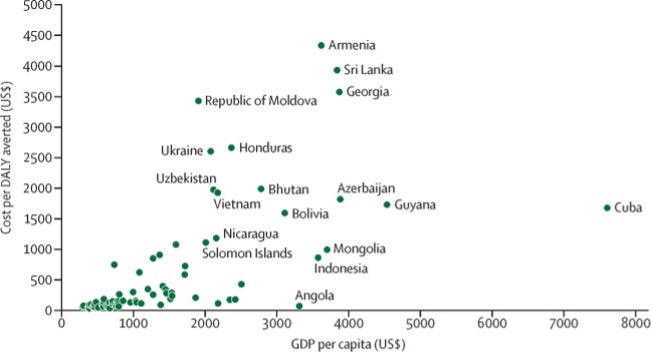
Figure Data from Table 4 of the article below: Debellut, F., Clark, A., Pecenka, C., Tate, J., Baral, R., Sanderson, C., … & Atherly, D. (2019). Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. The Lancet Global Health, 7(12), e1664-e1674. DOI: https://doi.org/10.1016/S2214-109X(19)30439-5
Another modelling study covered 63 middle-income countries not eligible for Gavi funding. It estimated that over the period of 2020-2029, rotavirus vaccines in these countries could avert 77 million cases of rotavirus gastroenteritis (RVGE) and 21 million clinic visits, 3 million hospitalizations, and 37,900 deaths due to RVGE. Using 0.5 times the per capita GDP of each country as the threshold for cost-effectiveness, the study found rotavirus vaccination was cost-effective in 77% of the middle-income countries (Figure 5.2) from a government perspective2. In addition, a systematic review in high-income regions compared using pentavalent and monovalent rotavirus vaccines with no intervention, which showed rotavirus vaccination was cost-effective under the willingness-to-pay thresholds compared to no vaccination3.
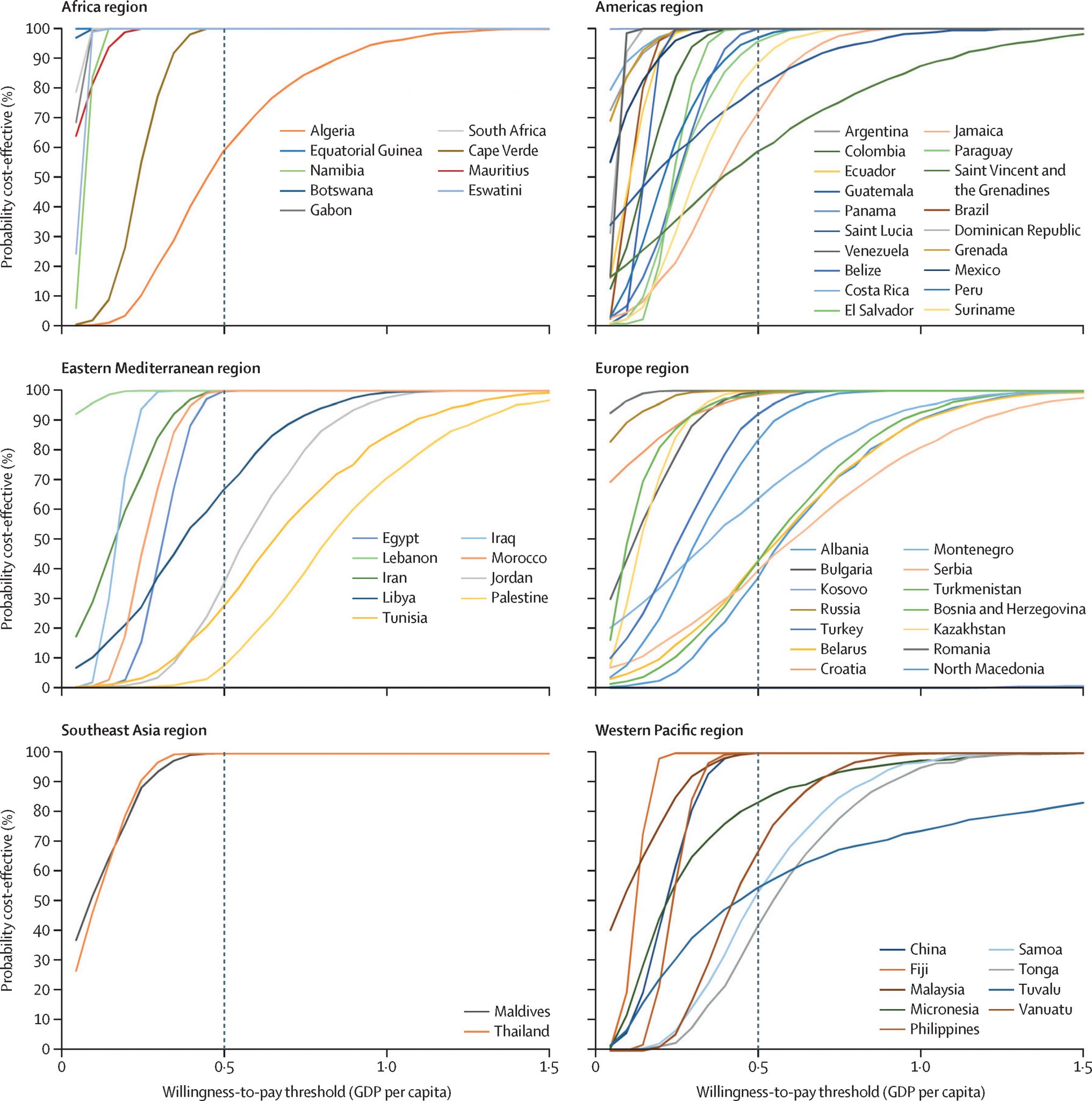
Source:Debellut, F., Clark, A., Pecenka, C., Tate, J., Baral, R., Sanderson, C., … & Atherly, D. (2021). Evaluating the potential economic and health impact of rotavirus vaccination in 63 middle-income countries not eligible for Gavi funding: a modelling study. The Lancet Global Health, 9(7), e942-e956. DOI: https://doi.org/10.1016/S2214-109X(21)00167-4
China Studies
Rotavirus vaccines have not been introduced into China’s National Immunization Program (NIP). However, economic evaluations indicate that including the rotavirus vaccines into NIP would reduce disease burden and is cost-effective. Moreover, a decrease in vaccine prices would further enhance their cost-effectiveness, especially for RotaTeq or Rotarix (Rotarix has not been launched in China market).
A study assessed the health impact and cost-effectiveness of using three vaccines – RotaTeq, Rotarix, and LLR, compared to the status quo, following a 2019 birth cohort in China. Results suggested that including the three vaccines into the NIP could reduce number of RVGE cases by 20.3% (LLR), 48.7% (Rotarix), and 62.6% (RotaTeq), and number of deaths by 22.4%(LLR), 63.2%(Rotarix), and 72.6% (RotaTeq). The national vaccination program, especially using RotaTeq or Rotarix showed to be cost-effective compared with the national GDP per capita. Future focus should address vaccine pricing and adopting vaccines with better efficacy4. Similarly, another study based on a hypothetical cohort of 100,000 infants found that both RotaTeq and Rotarix demonstrated excellent cost-effectiveness compared to LLR, with ICERs below the per capita GDP. It recommended replacing LLR with RotaTeq for large-scale vaccination5. A study in Zhejiang Province, where the economic development level is higher than the national average, found that introducing LLR and RotaTeq into the provincial immunization program was cost-effective compared to no intervention 6,7.
Table 5.1 Cost-effectiveness Analysis of Introducing Rotavirus Vaccines into the Immunization Programs
| Study | Vaccine Types | Comparison Scenario | Study Sample | Model and Time Horizon | ICER from a Societal Perspective (USD/QALY or DALY) | Comparison Threshold (Per Capita GDP, USD) | Cost-Effectiveness |
| Wang et al.*4 | LLR | self-funded Vaccination | 2019 Birth Cohort in China | Decision Tree-Markov State Transition Model Tracking Study Cohort Simulation over 5 Years | 26,759 | 10,276 | No |
| Rotarix | 9,503 | Yes | |||||
| RotaTeq | 8,833 | Yes | |||||
| Cui et al.*5 | Rotarix | LLR Vaccination | Hypothetical Cohort of 100,000 Infants | 2,105 | 7,594 | Yes | |
| RotaTeq | 1,715 | Yes | |||||
| Lv et al.**6 | LLR | No Vaccination | Zhejiang Province’s 2021 Birth Cohort | 4,632 | 16,866 | Yes | |
| RotaTeq | 512 | Yes | |||||
| Gan et al.**7 | RotaTeq | No Vaccination | Cohort of 1 Million Infants and Young Children Born in Zhejiang Province in 2019 | 3,358 | 15,575 | Yes |
Notes: *ICER: Cost per additional quality-adjusted life year (QALY) gained. **ICER: Cost per DALY averted.
Additionally, a cost-benefit analysis based on China’s 2019 birth cohort indicated that introducing RotaTeq into the NIP could prevent 5.68 million cases of rotavirus infection and save a social cost of US$1.1 billion. A vaccine price of $17.55 per dose was identified as the tipping point for achieving cost savings through the NIP introduction 8.
In 2023, the trivalent rotavirus vaccine LLR3, produced by Lanzhou Institute of Biological Products Co., Ltd., was approved in China. Its price and number of virus strains’ coverage are between the monovalent LLR and pentavalent RotaTeq vaccines. The economic evaluation studies of LLR3 are very limited.
Content Editor: Menglu Jiang, Zhangyang Pan
Page Editor: Ziqi Liu
References
1. Debellut, F., Clark, A., Pecenka, C., Tate, J., Baral, R., Sanderson, C., … & Atherly, D. (2019). Re-evaluating the potential impact and cost-effectiveness of rotavirus vaccination in 73 Gavi countries: a modelling study. The Lancet Global Health, 7(12), e1664-e1674. DOI: https://doi.org/10.1016/S2214-109X(19)30439-5
2. Debellut, F., Clark, A., Pecenka, C., Tate, J., Baral, R., Sanderson, C., … & Atherly, D. (2021). Evaluating the potential economic and health impact of rotavirus vaccination in 63 middle-income countries not eligible for Gavi funding: a modelling study. The Lancet Global Health, 9(7), e942-e956. DOI: https://doi.org/10.1016/S2214-109X(21)00167-4
3. Jesudason T, Rodarte A, Tordrup D, Carias C, Chen YH. Systematic review of rotavirus vaccination cost-effectiveness in high income settings utilising dynamic transmission modelling techniques. Vaccine. 2023 Jul 19. https://doi.org/10.1016/j.vaccine.2023.06.064
4. Jiahao Wang, Haijun Zhang, Haonan Zhang & Hai Fang (2022) Public health impact and cost-effectiveness of rotavirus vaccination in China: Comparison between private market provision and national immunization programs, Human Vaccines & Immunotherapeutics, 18:7, DOI: 10.1080/21645515.2022.2090162
5. Cui S, Tobe RG, Mo X, Liu X, Xu L, Li S. Cost-effectiveness analysis of rotavirus vaccination in China: Projected possibility of scale-up from the current domestic option. BMC infectious diseases. 2016 Dec;16:1-0.
6. Lv, H., Chen, F., Wang, Y., Chen, Y., & Hu, Y. (2023). Cost-effectiveness analysis of introducing rotavirus vaccine into immunization program in Zhejiang province, China: A decision tree-Markov model study. Human Vaccines & Immunotherapeutics, 19(2). https://doi.org/10.1080/21645515.2023.2217075
7. K, G., Z, J., Z. Y..et, al. Health economic evaluation for pentavalent rotavirus vaccine among children under 5 years old based on decision tree Markov model)[J]. International journal of epidemiology and infectious disease ,2023,50(06):404-409. DOI:10.3760/cma.j.cn331340-20230427-00069
8. Chen S, Gao S, Li J, Li J, Duan ZJ. Cost-benefit analysis of rotavirus vaccine included in the national immunization program in China. Vaccine. 2023 Jan 9;41(2):547-54.





