Global RV Vaccine Coverage
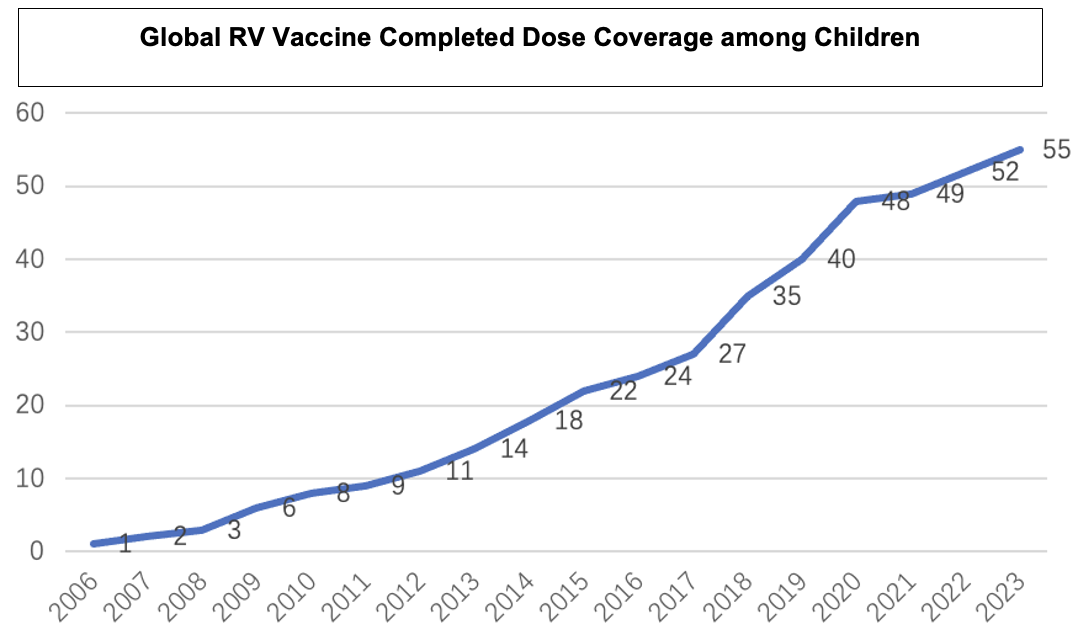
Data indicates that the global coverage of full-dose RV vaccination among children has been increasing annually, reaching 55% in 20231.Countries that have included the RV vaccine in their national immunization programs (NIPs) have higher vaccination coverage rates than the global average. In 2023, the average coverage for the first dose was 90.6%, while the coverage for the full dose was 83.6%1,2.
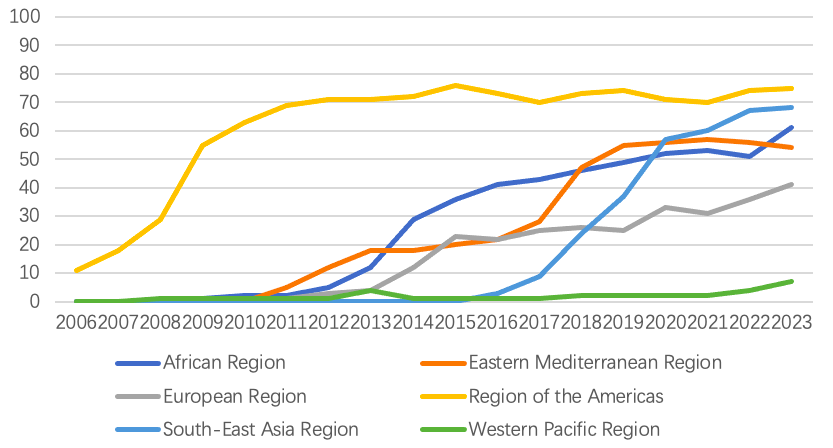
Regional data shows that the Americas have maintained a vaccination rate of over 70% since 2010, while the Western Pacific region has the lowest rate, at only 7% in 20231. Barriers such as the lack of data and research on the efficacy and safety of the RV vaccine, insufficient understanding of the risk-benefit about RV vaccination, and funding challenges for large-scale vaccination programs hindered the successful implementation of RV vaccination in Asia and the Pacific region3. Notably, since 2020, the full-dose vaccination rate for RV in children in Southeast Asia has significantly increased, reaching 68% by 20231. This increasing trend may be closely related to policy change in high density population countries in the region, such as Vietnam and Thailand, which included the RV vaccine in their NIPs or launched in pilot regions, starting in 20204,5.
(Data source: WHO Immunization Data portal)
RV Vaccine Coverage in China
Current Status of RV Vaccination in China
Currently, there is a lack of publicly available statistical data on RV vaccination in China. Research indicates that the vaccination coverage rate among Chinese children is generally low, particularly for full-dose coverage. A cross-sectional study conducted from August 5 to October 16, 2019, across 10 provinces (Beijing, Chongqing, Gansu, Guangdong, Henan, Jiangxi, Jilin, Shandong, Shanghai, and Yunnan) found that the first-dose vaccination rate for RV among children aged 6 months to 5 years was 20.3%, while the third-dose coverage was only 1.8% – significantly lower than the global average6. Similar coverage levels have been observed in other studies. For example, a stratified cluster random sampling survey of 8,400 children born between January 2008 and October 2012 in Guangzhou found that only 2,122 children (approximately 25.3%) had received at least one dose of the Lanzhou lamb RV (LLR) vaccine. Among these children, 1,904 (89.7%) had received only one dose, while 208 (9.8%) and 10 (0.5%) had completed two or three doses, respectively7. In Shenzhen’s Bao’an District, a study found that 32.3% of children born between 2014 and 2016 had received at least one dose of the domestic-produced (LLR) vaccine, with only 1.4% completing all three doses 8.
Research shows that since the introduction of the pentavalent RV vaccine (RV5, RotaTeq) in 2018, the LLR vaccination rate in Beijing and Shanghai has declined. The RV5 vaccination rates have been increasing annually and now far exceed those of LLR. In Beijing, the first-dose vaccination rate for RV5 in 2022 was 47.48%, compared to 6.74% for LLR; the third dose for RV5 was 44.12%, while only 0.03% for LLR9. In Shanghai’s Minhang District, the LLR vaccination rate had been increasing annually before 2018, with a first-dose rate of 66.0% and a third-dose rate of 43.2% in 2018. However, by 2020, the first-dose rate for LLR had dropped to 11.8%, with no third-dose vaccinations, while the first-dose rate for RV5 and the third-dose rate reached 42.3% and 29.8%10. Limited studies were conducted in other less developed regions. Further investigation is needed to map the RV vaccine coverage status.
Additionally, inequities in RV vaccination persists. A study conducted across 6 provinces in China from 2008 to 2012 found significant disparities in the first-dose LLR vaccination rate among families in different income levels: the vaccination rates were 45.0%, 37.7%, and 15.5% in high-, middle-, and low-income areas, respectively9. A study conducted in 2022 in Zhejiang and Henan on the vaccination status of migrant and left-behind children found that their RV vaccination rates were lower than those of local children. The vaccination rate for local urban children was 63.3%, with 43.5% for migrant children, 27.5% for non-left-behind children, and only 16.9% for left-behind children12. Expert consensus indicates that regions with relatively high RV vaccination rates are primarily located in economically developed areas or cities13.
Lot Release of RV Vaccine in China
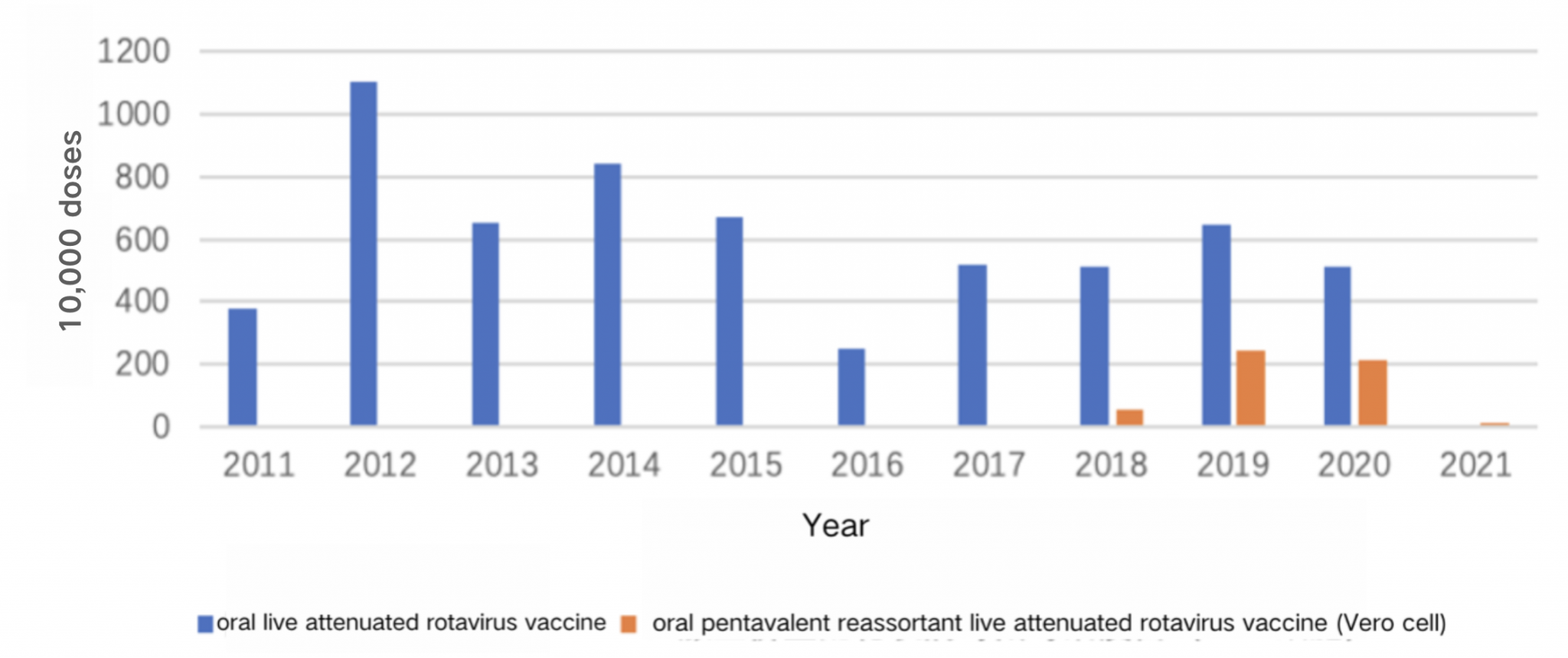
The lot release data showed that the domestic-produced LLR are predominant in the market. The LLR vaccine, produced by the Lanzhou Institute of Biological Products, has been in use for many years, estimated 5 to 7 million doses per year. The pentavalent RV vaccine produced by Merck Sharp & Dohme (MSD) gained approval in April 2018 and received lot release in September of the same year. By 2020, 3.99 million doses had been distributed, slightly lower than the 2019 level, accounting for approximately 37% of the market 15. According to incomplete statistics from the National Institutes for Food and Drug Control, the distribution of monovalent and pentavalent RV vaccines in China is shown in Figure 8.3 (data on trivalent domestic vaccines are not yet available).
(Data source: the National Institutes for Food and Drug Control)
Strategies to Improve RV Vaccine Coverage
Macro-Level Policy Interventions
Vaccine Supply and Local Vaccine Development
Countries with high birth rates, such as China, Vietnam, and Indonesia, often begin large-scale promotion of new vaccines only when domestically produced vaccines are ready and supply is sufficient. Increased availability of locally produced RV vaccines may expand and sustain coverage in these countries16. For example, the Indian government began introducing the RV vaccine in phases since 2016, with partial support from Gavi, achieving nationwide coverage by 2019. The development, clinical testing, and licensing of two locally produced vaccines—Rotavac® and Rotasiil®—were key factors in this successful introduction17.
Domestic research has used the Delphi method to establish an indicator evaluation system for prioritizing vaccines for inclusion in the NIP, with vaccine supply capacity being a critical factor considered by experts. “At least three domestic manufacturers” is one of the criteria for selecting vaccines for inclusion in the NIP18. However, China lacks research providing timely forecasts of supply and demand for non-NIP vaccines. Most provinces estimate future vaccine demand based on historical record of demand, leading to imbalances in vaccine supply and demand19.
Local Pilot Programs for RV Vaccine Introduction
Before nationwide introduction, small-scale pilot programs in specific regions can provide valuable experience for expanding the program later on. For example, in Pakistan, the introduction of the RV vaccine was initiated by the Punjab provincial government. With strong political leadership, the province used its own funds to pilot the vaccine in six districts starting in 2016. After Pakistan received Gavi support for nationwide rollout, the vaccine was introduced in other parts of Punjab province in 2017. This phased approach provided time for other regions to expand cold chain capacity and prepare for the vaccine’s introduction, contributing to the successful nationwide rollout17.
In China, whether non-NIP vaccines are included in provincial immunization programs is also an important consideration when introducing new vaccines18. However, no pilot programs have yet been implemented to include the RV vaccine in local immunization programs. In terms of financing, 14 provinces have included the RV vaccine and three other non-NIP vaccines (pneumococcal vaccine, human papillomavirus vaccine, and Haemophilus influenzae type b vaccine) in the reimbursement scope of urban employee medical insurance personal accounts. Most of these provinces have established family pooling accounts, allowing insured individuals and their family members to use these accounts to pay for non-NIP vaccines20. Provincial-level coverage has been achieved in Fujian, Zhejiang, Guizhou, Guangxi, Tibet, Chongqing, and Yunnan, while Jiangsu (Nanjing, Suzhou, Yangzhou, Taizhou, and Xuzhou), Guangdong (Shenzhen, Zhuhai, Guangzhou, Foshan, Huizhou, and Meizhou), Henan (Zhengzhou), Shaanxi (Xi’an), Hunan (Changsha), Shandong (Qingdao), and Liaoning (Shenyang), only implemented the policy in selected cities.
Interventions Targeting Healthcare Providers
Training and Education for Healthcare Providers
The underestimation of the severity of RV by public health sector is a global norm, particularly in low- and middle-income countries. Research suggests that educational programs targeting healthcare providers and caregivers should be promoted as a primary intervention to increase RV vaccination rates, with medical professional associations and public health authorities facilitating the education campaign for caregivers and physicians21. However, significant gaps remain in studies of healthcare providers’ education intervention.
Improving Communication During Vaccination
Globally, changing the way clinicians communicate about vaccines has been shown to effectively increase vaccination rates. For example, providing more detailed vaccine information and using a “presumptive” communication style, such as asking, “Your child has some vaccines due today,” rather than, “What vaccines would you like today?”22.
In China, a study found that implementing a vaccination notification system significantly increased vaccination rates at the vaccination clinics in Changchun. The notification methods included clearly informing vaccine recipients or their caregivers of the vaccine’s name, manufacturer, administration process, price, indications, contraindications, immunization schedule, potential adverse reactions, and how to deal with the reactions. Additionally, caregivers were required to read and sign an informed consent form, confirming they fully understand the information given and providing their signature 23. In another randomized controlled trial, healthcare providers in the intervention group used shared decision-making (SDM) and patient decision aids (PDAs) to provide comprehensive information on RV, its clinical manifestations, vaccine knowledge, vaccination timing, benefits, efficacy, side effects, and costs. The control group used traditional methods. The intervention group’s vaccination rate was 16.7% higher than the control groups, demonstrating that SDM combined with PDAs, by providing richer information, helps families make more informed decisions, thereby effectively increasing RV vaccination uptakes24.
Interventions Targeting Vaccine Recipients
Vaccination Reminders
Although global research on the effectiveness of using reminder to increase the RV vaccination rate is limited, interventions providing vaccine information to parents through websites and social media (e.g., text messages, phone calls, emails) have been shown to effective in improving childhood immunization rate 22. Specifically, studies suggest that centralized reminders or recall systems for early childhood vaccinations are more cost-effective than traditional reminders from healthcare providers, especially when the reminders include the child’s name25,26.
Similarly, domestic studies on interventions for other non-NIP vaccines suggest that app-based reminders sent to parents via web and mobile devices can also improve non-NIP vaccination rates. An experimental study in Chongqing showed that sending vaccine knowledge, real-time inventory, post-vaccination adverse reaction management, and vaccination reminders to caregivers via an app positively improved migrant children’s caregivers’ vaccine knowledge, attitudes, and vaccination willingness27. Though another study found no significant difference in vaccination rate increases between reminders sent by village doctors via smartphone apps and the traditional text message reminders, the introduction of intelligent information systems helps village doctors manage children’s vaccination status and their parents’ information28.
Prenatal Health Education
Both domestic and international studies have shown that prenatal health education for pregnant women positively impacts infant vaccination rates and maternal vaccine knowledge. A study in Japan provided comprehensive vaccine-related health guidance to women in the third trimester and 3 to 6 days postpartum, covering vaccine types, disease prevention, vaccine efficacy, side effects, and vaccination schedules. The intervention significantly increased infant vaccination rates within three months (34.3% in the intervention group vs. 8.3% in the control group). However, there was no significant difference between prenatal and postpartum intervention groups29.
In a study in Zhejiang, China, pregnant women over 12 weeks’ gestation received prenatal vaccine education, while the control group did not. The intervention group showed significantly improved vaccine knowledge, and their children were more likely to take vaccines, complete the full-course vaccination, and have better timeliness in vaccination compared to the control group30. What’s more, considering the high coverage of prenatal care in China, implementing prenatal education can better ensure the dissemination of vaccine health education and equip parents with the necessary knowledge before newborn vaccination. This study recommends that vaccine education should start during pregnancy30.
Content Editor: Menglu Jiang, Ziqi Liu
Page Editor: Ziqi Liu
References
- World Health Organization. RV immunization coverage. World Health Organization. Retrieved from https://immunizationdata.who.int/global/wiise-detail-page/RV-vaccination-coverage?CODE=Global&ANTIGEN=ROTAC&YEAR=
- World Health Organization. Indicator metadata registry: RV vaccination coverage. Retrieved November 3, 2024, from https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4758
- Buchy, P., Chen, J., Zhang, X. H., Benninghoff, B., Lee, C., & Bibera, G. L. (2021). A review of RV vaccine use in Asia and the Pacific regions: challenges and future prospects. Expert Review of Vaccines, 20(12), 1499–1514. https://doi.org/10.1080/14760584.2020.1853532
- Charoenwat, B., Suwannaying, K., Paibool, W. et al. The impact of RV vaccination on acute diarrhea in Thai children under 5 years of age in the first year of universal implementation of RV vaccines in the National Immunization Program (NIP) in Thailand: a 6-year analysis. BMC Public Health 23, 2109 (2023). https://doi.org/10.1186/s12889-023-16958-0
- Le LK, Pham TP, Mai LT, Nguyen QT, Tran MP, Ho TH, Pham HH, Le SV, Hoang HN, Lai AT, Huong NT. Intussusception and Other Adverse Event Surveillance after Pilot Introduction of RV Vaccine in Nam Dinh and Thua Thien Hue Provinces—Vietnam, 2017–2021. Vaccines. 2024 Feb 7;12(2):170. https://doi.org/10.3390/vaccines12020170
- Zhang H, Lai X, Mak J, Sriudomporn S, Zhang H, Fang H, Patenaude B. Coverage and Equity of Childhood Vaccines in China. JAMA Netw Open. 2022;5(12):e2246005. doi:10.1001/jamanetworkopen.2022.46005
- He Q, Wang M, Xu J, Zhang C, Wang H, Zhu W, Fu C. RV vaccination coverage among children aged 2-59 months: a report from Guangzhou, China. PloS one. 2013 Jun 28;8(6):e68169. https://doi.org/10.1371/journal.pone.0068169
- Tang G, Chen Y, Li Y, et al. Investigation on the utilization of domestic RV vaccine in Bao’an District, Shenzhen. International Journal of Virology,2020,27(05) : 417-417. DOI: 10.3760/cma.j.issn.1673-4092.2020.05.015
- Yuan Q, Cao Z, Ji W, Yu R, Miao L, Wen X, … & Suo L. (2024). Analysis of RV vaccination status among children born in Beijing from 2017 to 2022. Modern Preventive Medicine (15),2770-2773.doi:10.20043/j.cnki.MPM.202403255.
- Lu J, Guan B, Zhang L, Mei K, Lu X, & Lu Y. (2021). RV vaccination coverage among children born in 2013-2020 in Minhang District, Shanghai. Chinese Journal of Vaccines and Immunization (06),695-699.doi:10.19914/j.CJVI.2021127.
- Liu Y, Yue C, Li Y, et al. Analysis of the vaccination status of LLR strain live attenuated RV vaccine among children in six provinces of China. Chinese Journal of Preventive Medicine,2018,52(3) : 282-282. DOI: 10.3760/cma.j.issn.0253-9624.2018.03.012
- Zhou Y, Li D, Cao Y, Lai F, Wang Y, Long Q, Zhang Z, An C, Xu X. Immunization coverage, knowledge, satisfaction, and associated factors of non-National Immunization Program vaccines among migrant and left-behind families in China: evidence from Zhejiang and Henan provinces. Infectious Diseases of Poverty. 2023 Oct 10;12(05):69-80. https://mednexus.org/doi/full/10.1186/s40249-023-01145-5
- Expert Consensus Writing Group on RV Gastroenteritis. Expert consensus on immunoprophylaxis of RV gastroenteritis in children (2024 edition). Chinese Journal of Preventive Medicine, 2024,58(00):1-33. DOI:10.3760/cma.j.cn112150-20231220-00472
- Li L, Liu N, Huang R, et al. Analysis of domestic RV vaccination in Chengdu. International Journal of Virology,2020,27(04) : 313-313. DOI: 10.3760/cma.j.issn.1673-4092.2020.04.012
- Ping An Securities. (2021, February 5). Review of key vaccine batch release data: Total volume increases, structural upgrades significant.https://pdf.dfcfw.com/pdf/H3_AP202102051459184106_1.pdf?1612539184000.pdf
- Deen J, Lopez AL, Kanungo S, Wang XY, Anh DD, Tapia M, Grais RF. Improving RV vaccine coverage: Can newer-generation and locally produced vaccines help? Hum Vaccin Immunother. 2018 Feb 1;14(2):495-499. doi: 10.1080/21645515.2017.1403705. Epub 2017 Dec 21. PMID: 29135339; PMCID: PMC5806648.
- Rota Council: RV VACCINE INTRODUCTION AND COVERAGE https://publichealth.jhu.edu/sites/default/files/2024-02/rota-brief1-introduction2022-1ax.pdf
- Ma, C.; Li, J.; Wang, N.; Wang, Y.; Song, Y.; Zeng, X.; Zheng, C.; An, Z.; Rodewald, L.; Yin, Z. Prioritization of Vaccines for Inclusion into China’s Expanded Program on Immunization: Evidence from Experts’ Knowledge and Opinions. Vaccines 2022, 10, 1010.
- Peixi Dai, Qing Wang, Mengmeng Jia, Zhiwei Leng, Shuyun Xie, Luzhao Feng & Weizhong Yang (2023) Driving more WHO-recommended vaccines in the National Immunization Program: Issues and challenges in China, Human Vaccines & Immunotherapeutics, 19:1, DOI: 10.1080/21645515.2023.2194190
- Zhong G, Wang M, Ge J, et al. Analysis of the implementation of payment policies for four non-National Immunization Program vaccines in China. Chinese Journal of Preventive Medicine,2023,57(11):1843-1847. DOI:10.3760/cma.j.cn112150-20230118-00043.
- Vecchio AL, Liguoro I, Dias JA, Berkley JA, Boey C, Cohen MB, Cruchet S, Salazar-Lindo E, Podder S, Sandhu B, Sherman PM. RV immunization: Global coverage and local barriers for implementation. Vaccine. 2017 Mar 14;35(12):1637-44.
- Cataldi J, Kerns M, O’Leary S. Evidence-based strategies to increase vaccination uptake: a review. Current Opinion in Pediatrics. 2020; 32 (1): 151-159. doi: 10.1097/MOP.0000000000000843.
- Tao Y, Zheng T, Tao Y. Analysis of oral RV vaccine coverage and vaccine awareness before and after the implementation of the vaccination notification system in Changchun. Chinese Journal of Biologicals,2020,33(7):845-848.
- Lin SC, Tam KW, Yen JY, Lu MC, Chen EY, Kuo YT, Lin WC, Chen SH, Loh EW, Chen SY. The impact of shared decision making with patient decision aids on the RV vaccination rate in children: A randomized controlled trial. Preventive Medicine. 2020 Dec 1;141:106244.
- Kempe, A., Saville, A. W., Beaty, B., Dickinson, L. M., Gurfinkel, D., Eisert, S.,& Herlihy, R. (2017). Centralized reminder/recall to increase immunization rates in young children: how much bang for the buck?. Academic pediatrics, 17(3), 330-338.
- Kempe, A., Saville, A. W., Dickinson, L. M., Beaty, B., Eisert, S., Gurfinkel, D., … & Herlihy, R. (2015). Collaborative centralized reminder/recall notification to increase immunization rates among young children: a comparative effectiveness trial. JAMA pediatrics, 169(4), 365-373. doi:10.1001/jamapediatrics.2014.3670
- Jiawei Xu, Wenge Tang, Wei Qiu, Yuan Yao, Ning Yao, Jianghong Zhong, Xiang Zhu & Qing Wang (2022) Effects of mobile APP for immunization on vaccination compliance of migrant children in southwest China: A community trial study, Human Vaccines & Immunotherapeutics, 18:7, DOI: 10.1080/21645515.2022.2135853
- Chen L, Du X, Zhang L, van Velthoven MH, Wu Q, Yang R, Cao Y, Wang W, Xie L, Rao X, Zhang Y, Koepsell JC. Effectiveness of a smartphone app on improving immunization of children in rural Sichuan Province, China: a cluster randomized controlled trial. BMC Public Health. 2016 Aug 31;16(1):909. doi: 10.1186/s12889-016-3549-0. PMID: 27581655; PMCID: PMC5006404.
- Saitoh, A., Nagata, S., Saitoh, A., Tsukahara, Y., Vaida, F., Sonobe, T., … & Murashima, S. (2013). Perinatal immunization education improves immunization rates and knowledge: a randomized controlled trial. Preventive medicine, 56(6), 398-405.
- Hu Y, Chen Y, Wang Y, Song Q, Li Q. Prenatal vaccination education intervention improves both the mothers’ knowledge and children’s vaccination coverage: Evidence from randomized controlled trial from eastern China. Hum Vaccin Immunother. 2017 Jun 3;13(6):1-8. doi: 10.1080/21645515.2017.1285476. Epub 2017 Feb 21. PMID: 28319453; PMCID: PMC5489276.





