PCV13
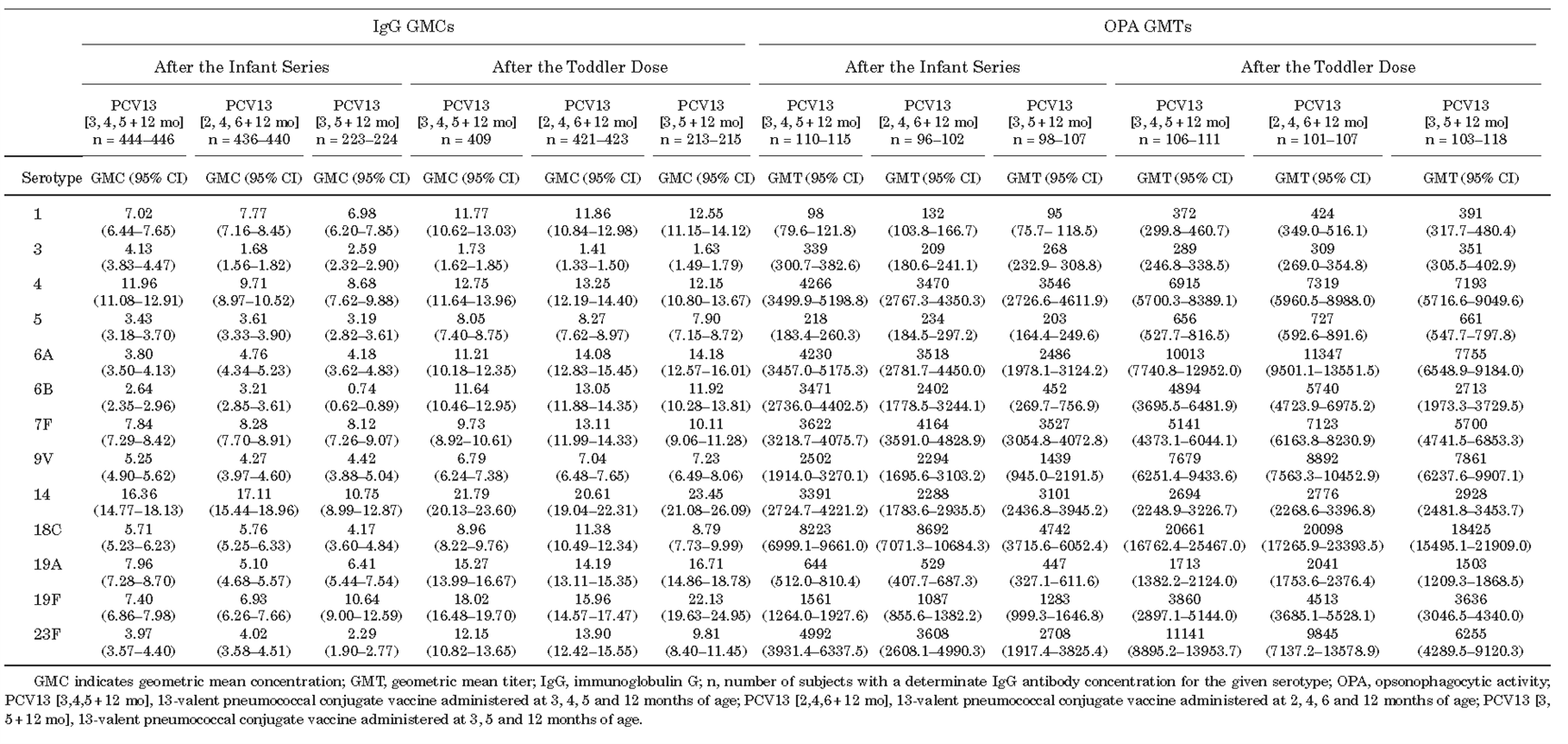
A clinical trial enrolling 1196 infants found that in healthy Chinese infants, PCV13 administered as a 3- or 2-dose infant series and with a subsequential toddler booster demonstrated good immunogenicity, tolerability, and protective effect against PCV13-covered serotypes. However, the IgG geometric mean concentrations (GMCs) were lower for some serotypes (e.g., 6B, 14, 18C, and 23F) in the 2-dose schedule group1 (Table 1 and 2).
Table 1 Percentage of IgG Responders (IgG ≥ 0.35 μg/mL) and OPA Responders (GMT ≥ LLOQ) One Month After Completion of the Infant Series and Toddler Dose of PCV13(LLOQ = Lower Limit of Quantification)
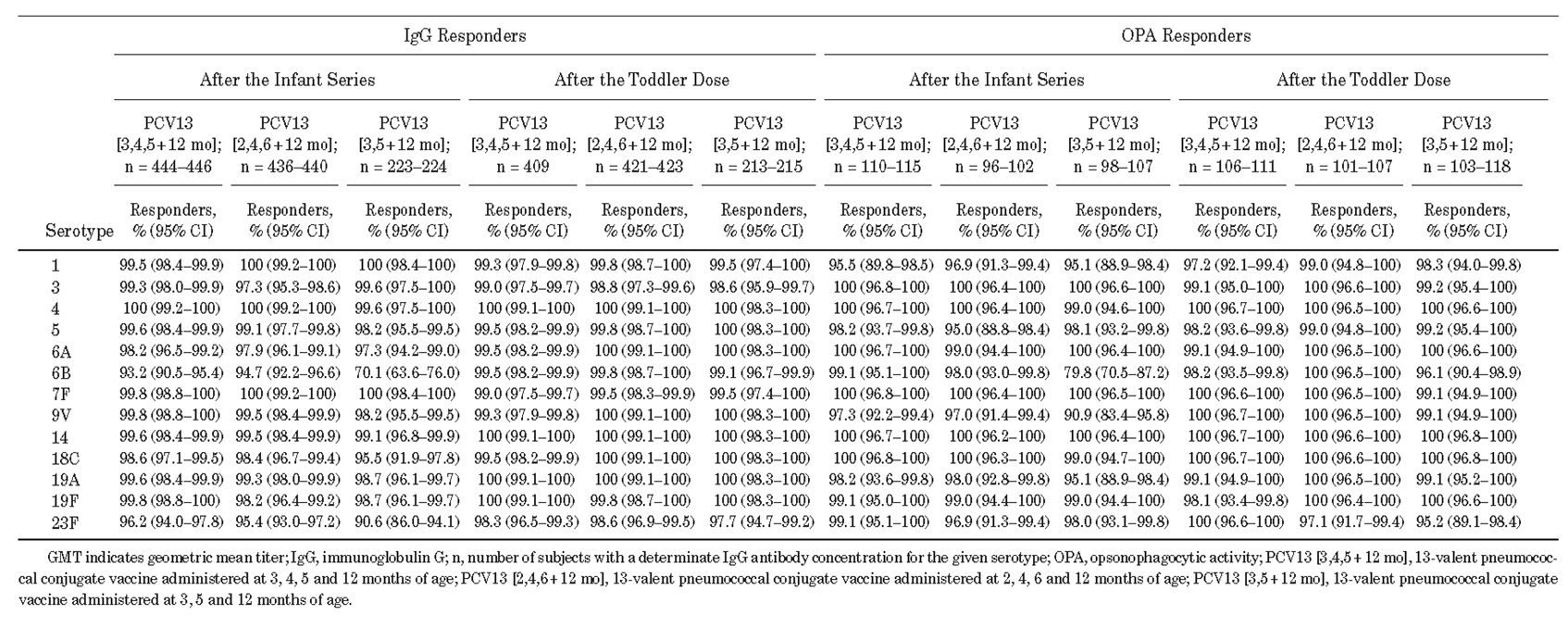
Table 2 IgG GMC and OPA GMT One Month After Completion of the Infant Series and Toddler Dose of PCV13
Another clinical study explored the immune response of PCV13 among PPSV23-naïve adults aged 18 to 49. The study included a total of 1316 study participants and grouped by age. It found that the youngest subgroup (18-29 years) had the highest immune response. Localized reactions and systemic events were more common and self-limiting in adults aged 18-49 years compared to the 60-64 age group. GMT of OPA antibodies were significantly higher in 18-49 age group compared to those in the 60-64 age group across almost all serotypes except serotype 3. The highest GMTs of OPA antibodies were observed in the youngest subgroup (18-29 years of age); the lowest GMTs of OPA antibodies were observed in adults 60-64 years of age. Adults 18-49 years had significantly higher IgG-GMCs for all serotypes—except serotypes 3, 5, 7F, 9V, and 18C, than those aged 60-64 years. For adults aged 18-49 years, GMTs of OPA-antibodies were significantly higher for all serotypes at 1-year postvaccination than pre-vaccination. In all age groups and subgroups, antibody response curves for GMTs of OPA antibodies measured before, 1 month after, and 1 year after vaccination. The findings showed higher antibody responses for all serotypes at 1 month after vaccination compared with pre-vaccination; the immune responses diminished at 1 year but remained higher than the pre-vaccination titers. For all serotypes (except serotype 3), OPA response curves were higher in adults aged 18-49 years than in adults aged 60-64 years. In subgroups, adults aged 18-29 years had the highest OPA response curves and adults aged 40-49 years had the lowest OPA response curves2.
A Swedish clinical study also found that adverse reactions to PCV13 vaccination were mostly localized reaction with mildly or moderately severity in tenderness, pressure, or fever. The most common reported adverse events in the group were infections and infestations (e.g., gastroenteritis and nasopharyngitis) followed by gastrointestinal disorders 3.
Meta-analysis results showed that compared with other pneumococcal conjugate vaccines, PCV13 was associated with a lower tenderness symptom, but a higher incidence of swelling and fever. There were no statistically significant differences in the incidence of redness, loss of appetite, or irritability4.
Studies have shown that initiating the PCV13 vaccination schedule at 6 weeks of age provides strong immunogenicity, efficacy, and safety in infants and young children. Moreover, this immunization program offers substantial protective effects for both children and adults, significantly reducing the incidence of invasive pneumococcal disease (IPD), lowering the nasopharyngeal colonization rate of pneumococcus, and decreasing pneumococcal antimicrobial resistance5.
There are studies focusing on the comparison of immunogenicity between different immunization schedules. For example, it was found that children who completed the full course of PCV using either a 2-dose (2p) or 3-dose (3p) primary series were able to obtain good Antibody Positive Rate (APR). However, the 2-dose schedule did not elicit adequate immune responses to serotypes 6B and 23F 6. Except for the antigen of serotype 6B, no significant differences in immunogenicity were observed between the 2p+1 (two primary doses plus one booster) and 3p+1 (three primary doses plus one booster) schedules. It is therefore recommended that the 3-dose primary immunization schedule be adopted during the primary immunization stage in China and in regions with a high burden of IPD6.
PCV13 vs. PPV23
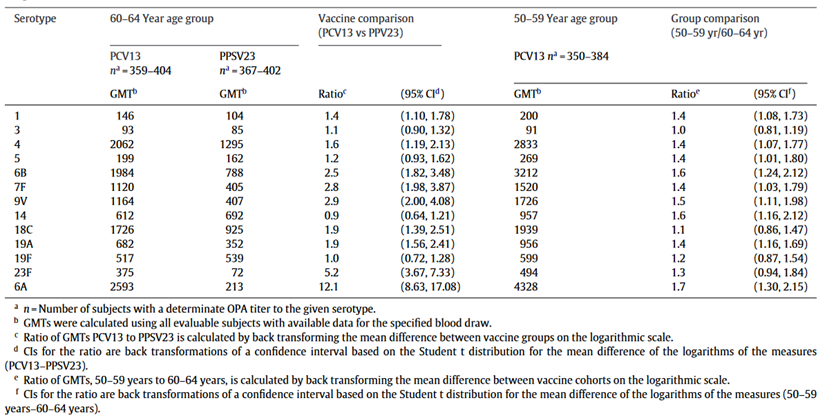
A clinical trial in the United States, which included 1234 subjects, compared the 1-dose PCV13 with PPV23 among pneumococcal vaccine naïve adults. The study found that PCV13 induced a stronger functional immune response than PPV23, suggesting that PCV13 has a stronger immune advantage than PPV23 in preventing vaccine-type pneumococcal infections7. The GMT of OPA in the PCV13 group in the first month after vaccination was significantly higher than that in the PPV23 group (Table 3). For most serotypes covered by PCV13, PCV13 induced a greater functional immune response than PPV23, suggesting that PCV13 has an immunologic advantage over PPV23 in preventing vaccine-type pneumococcal infections. Immune responses to PCV13 were typically greater in adults aged 50-59 years compared with adults aged 60-64 years. Antibody levels declined slowly over time after both types of vaccinations but remained twice as high as pre-vaccination levels after 1 year. Four to seven years after vaccination, antibody levels dropped below pre-vaccination levels. In subjects aged 60-64 years, the percentage of subjects with severe pain at the injection site after PPV23 vaccination was significantly higher than that for PCV13, whereas the percentage of subjects with mild pain after PCV13 vaccination was significantly higher than that for PPV23. A higher percentage of PCV13 subjects aged 50-59 reported pain and limited arm mobility when compared to the older age group, and no significant differences in systemic events were observed between the PCV13 and PPV23 groups. The incidence of adverse events was similar in PCV13 (17.0%) and PPV23 (16.7%) groups. The majority of adverse events consisted of diseases and conditions common among older adults, with infectious diseases being the most common type of reported adverse event in all groups7.
Table 3 Comparison of pneumococcal OPA GMTs 1 month after PCV13 and PPV23 immunization in subjects aged 60-64 years, and 1 month after PCV13 immunization in subjects aged 50-59 years
The research team also conducted a follow-up study to explore the immune response of having a booster dose after 4 years of the first PCV13/PPV23 injection. The 60–64 age group initially given PCV13 received PCV13 or PPSV23, and those initially given PPSV23 received another PPSV23. All adults aged 50–59 years were re-vaccinated with PCV13. The team compared the results of the OPA GMTs for different vaccination combination strategies before and one month after vaccination: in adults aged 50-64 years, subjects initially vaccinated with PCV13 established an immune state, and a secondary immune response could be induced by further vaccination with PCV13 or PPV23. The study also found that PCV13 induced stronger immune responses in younger adult groups (aged 50–59 years). If PCV13 is administered with an appropriate interval, the immune response can be sustained and may be expanded to non-PCV13 serotypes by the sequential PPV23 vaccination. In contrast, a second dose of PPV23 after an initial PPV23 vaccination generally elicited lower immune responses8. In another relevant study, the research team confirmed that among the pneumococcal vaccine–naïve adults 60–64 years of age, an initial PCV13 augmented the anti-pneumococcal response to subsequent administration of PPSV23 for many of the serotypes in common to both vaccines. However, initial PPSV23 resulted in a diminished response to subsequent administration of PCV13 for all serotypes. With a relatively short 1-year interval between doses, responses after a second vaccination with PCV13 (PCV13/PCV13) or PPSV23 (PCV13/PPSV23) were noninferior for a majority of serotypes compared with the initial PCV13 dose 9.
PPV23
A cohort study in Brazil found that the IgG-GMCs antibodies was significantly higher 1 month after vaccination than pre-vaccination and remained significantly higher 1 year after vaccination; except for serotype 5, the IgG-GMCs were comparable to the pre-vaccination level 1 year after vaccination. The mean IgG concentrations were higher in males than in females across all time 10.
A systematic review of PPV23 in China found that the immune response to PPV23 was attenuated in immunosuppressed populations such as patients with bone marrow or organ transplantation, inflammatory bowel disease, hematological neoplasms, chronic liver disease, patients on immunosuppressive therapy, and patients with chronic renal or respiratory disease11. However, the response would maintain among populations with underlying diseases but not immunosuppression such as those with no spleen, solid tumors, treatment with TNF-a suppression, and rheumatic disease 12. A study in China showed good immunogenicity of one dose of PPSV23 in COPD patients, with two-fold increase of antibody levels from 65.2% (serotypes 3) to 94.4% (serotype 2) after 4 weeks of vaccination 13. A study in the United States showed that PCV7 might induced a greater functional antibody response than PPV23 in patients with COPD that may persist for 2 years after vaccination14.
A China study showed that PPV23 induced a significant immune response with a 2-fold growth rate of antibody levels among the 23 serotypes ranged from 51.49% to 97.01%, while the growth rate of serotypes 8, 9N, 18C, and 33F was more than 90%15. The geometric mean of the specific antibodies to IgG was significantly higher among community elderly people who were vaccinated with PPV2316. The levels of functional antibodies to 23 serotypes were significantly higher in elderly people aged >60 years who were vaccinated with PPV23 compared with pre-vaccination, showing a good immunogenicity of PPV23; and the efficacy did not diminish with the increase of vaccine receivers’ age17. Miemyk et al. found that post-PPV23 vaccination, the IgG GMCs and OPA titers for serotypes 4, 6B, 14, and 19F increased significantly18. Serpa et al. further confirmed that age and repeat immunization did not negatively affect VH3-specific immunogenicity of PPV23 in middle-aged and elderly individuals 19.
Among immunocompetent adults and those with comorbidities but no severe immunodeficiency, the efficacy of PPV23 in preventing IPD ranged from 50% to 80%20. It also helped reduce the severity of pneumonia and the risk of mortality. Studies estimated the vaccine effectiveness against IPD to be between 52% and 74%21. Additionally, PPV23 vaccination significantly reduced the incidence of upper respiratory tract infections in older adults, with a protective efficacy against pneumococcal respiratory infections ranging from 40% to 80%22,23. One study by Xu Ying et al confirmed that PPV23 conferred protective, cost-effective, and safe outcomes in community-dwelling elderly individuals—particularly those with COPD and coronary heart diseases showing reductions of 69.7% in lower respiratory tract infections, 72.6% in antibiotic use, and 65.9% in hospitalization 24.
Antibody titers increased significantly in the short term after PPV23 vaccination for the older adults. Though evidence showed a decline in antibody levels over time, the IgG and functional antibody levels after PPSV23 in adults persist above concentrations in unvaccinated adults for at least 5–10 years in most studies 25,26. A study conducted in Shanghai found that PPV23 induced specific antibodies in older adults, with the highest seropositivity rate occurring three months after vaccination, and antibody levels persisting for at least six months27. While PPV23 is effective in preventing IPD, its protective efficacy wanes over time, with the most pronounced effect within three years and diminished after five years28. Another study found that the protective efficacy of PPV23 against pneumonia was 87.44% within six months of vaccination, and 85.83% within the first year29.
A study in Shanghai about the immunogenicity and safety of the PPV23 revaccination among people aged 60-70 found that after immunization, the total GMI of antibodies in the revaccination group was lower than that in the first vaccination group. The GMI of all serotypes in the revaccination group was lower than that in the first vaccination group 30.
A study in Shanghai, China demonstrated that PPV23 is safe for large-scale use in adults aged 60 and older. Between 2013 and 2017. The reported incidence of adverse events following immunization (AEFI) was 33.04 per 100,000 doses—significantly lower than the national average of 129.99 per 100,000 doses reported in 2016. Most adverse events were mild and resolved quickly with treatment31.
Another safety evaluation conducted in Guangzhou reported an AEFI incidence of 39.13 per 100,000 doses from 2010 to 2015, which were mainly fever and redness. Other AEs monitored were allergic rash, febrile convulsions, and angioedema. Five cases of febrile convulsions were reported as serious adverse reactions, all of which were resolved or improved with treatment. No rare or very rare adverse reactions were identified. The study also noted that in Guangzhou, PPV23 was predominantly administered to children, with relatively low coverage among the elderly 32.
Co-administration of PPV23 and Influenza Vaccines
A clinical trial in Jiangsu Province enrolling 1035 subjects found that co-administration of PPV23 and TIV did not affect the immunization efficacy of pneumococcal polysaccharide vaccine. The incidence of adverse reactions after vaccination was 15.9%. Most of the local adverse reactions were pain, swelling, and redness. Most of the systemic adverse reactions were abnormal temperature, nausea, and diarrhea, which were cured after symptomatic treatment. The study suggests that co-administration of PPV23 and TIV is both immunogenic and safe, and they can be administered simultaneously33.
A cohort study in Rizhao recruited 339 subjects and found that the incidence of upper respiratory tract infections in the vaccinated group (co-administered PPV23 and split-virus influenza vaccine, InfV-B) and the control group (neither vaccine were administered) were 5.3% and 13.3%, respectively, with a statistically significant difference. Co-administration of PPV23 and InfV-B reduced the incidence of upper respiratory tract infections, with a protective efficacy of 60%. In addition, the total cost of vaccination and related expenses was CNY 58,739, while the estimated benefit reached RMB 236,733.86. The benefit-cost ratio was 4.03, and the net benefit was 177,994.86 CNY. The study demonstrates that co-administration of PPV23 and InfV-B in community-dwelling older adults can reduce the incidence of upper respiratory infections and offers substantial cost-effectiveness34.
Another study on the incidence of adverse reactions after the co-administration showed that the overall rates of adverse reactions in each group were as follows: all participants, 10.13%; influenza vaccine alone, 5.35%; domestic PPV23 alone, 11.63%; imported PPV23 alone, 9.52%; co-administration group I (influenza vaccine + domestic PPV23), 17.24%; and co-administration group II (influenza vaccine + imported PPV23), 12.63%. Most reactions were local, such as injection site pain, and were mild in severity. All adverse reactions occurred within 7 days after vaccination, with the majority (82.61%) occurring between 30 minutes and 1-day post-vaccination. All cases resolved within 7 days, with 84.78% recovering within 1 day. The results suggest that the incidence of adverse reactions was higher in the co-administration groups than in the single vaccination groups. Among the single vaccination groups, the domestic PPV23 group had a higher reaction rate than the imported PPV23 group, which was in turn higher than the influenza vaccine group, although the differences were not statistically significant (P > 0.05). Similarly, among the co-administration groups, the group using domestic PPV23 had a higher reaction rate than the group using imported PPV23, but the difference was also not statistically significant (P > 0.05) 35.
Content editor: Siqi Jin, Ziqi Liu
Page Editor: Ziqi Liu
References
[1] Zhu, F., Hu, Y., Li, J., Ye, Q., Young, M. M., Liang, J. Z., … Scott, D. A. (2019). Immunogenicity and Safety of the 13-Valent Pneumococcal Conjugate Vaccine Administered in a 3 + 1 versus 2 + 1 Dose Schedule Among Infants in China. The Pediatric Infectious Disease Journal, 38(11), 1150–1158
2 Bryant K A,Frenck R,Gurtman A, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 18-49 years of age,naive to 23-valent pneumococcal polysaccharide vaccine .Vaccine, 2015, 33: 5854-5860.
3 Silfverdal Sven Arne,Flodmark Carl-Erik,Rombo Lars et al. 13-Valent pneumococcal conjugate vaccine (PCV13) in children partially immunized with 7-valent pneumococcal conjugate vaccine (PCV7): a phase 3, open-label trial.[J] .Vaccine, 2013, 31: 1284-92.
4 Sun J, Tang Q, Wang H. Meta analysis on the safety of 13-valent pneumococcal polysaccharide conjugated vaccine. Prac Prevent Medicine,2019,26(07):871-873.
5 Li Y, An Z, Wang H. Review on immunogenicity, safety and efficacy of 13-valent pneumococcal conjugate vaccine administered at 6 weeks of age in infants. Chinese Journal of Public Health, 2018, 34(11): 1491-1495.
6 Hu Y, Tang X, Guo J, Chen Y, Shen L. The Meta-Analysis of Immunological Effects of Pneumococcal Polysaccharide Conjugate Vaccine Following Different Immunization Schedules. Chinese Journal of Vaccines and Immunization,2014,20(03):245-249+278.
7 Jackson, L. A., Gurtman, A., Van Cleeff, M., Jansen, K. U., Jayawardene, D., Devlin, C., Scott, D. A., Emini, E. A., Gruber, W. C., & Schmoele-Thoma, B. (2013). Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine, 31(35), 3577–3584. https://doi.org/10.1016/j.vaccine.2013.04.085
8 Jackson L A, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older[J]. Vaccine, 2013, 31(35): 3594-3602.
9 Greenberg, R. N., Gurtman, A., Frenck, R. W., Strout, C., Jansen, K. U., Trammel, J., Scott, D. A., Emini, E. A., Gruber, W. C., & Schmoele-Thoma, B. (2014). Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine–naïve adults 60–64 years of age. Vaccine, 32(20), 2364–2374. https://doi.org/10.1016/j.vaccine.2014.02.002
10 Brandão Angela Pires,de Oliveira Tânia Cristina,de Cunto Brandileone Maria Cristina et al. Persistence of antibody response to pneumococcal capsular polysaccharides in vaccinated long term-care residents in Brazil.[J] . Vaccine, 2004, 23: 762-8.
11 Xu Y, Liu Y, An Z. Immune and protective effect of pneumococcal polysaccharide vaccine in elderly population: a review [J]. Chinese Journal of Public Health,2021,37(04):584-588.
12 Agarwal N, Ollington K, Kaneshiro M, Frenck R, Melmed GY. Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine. 2012 Feb 14;30(8):1413-24. doi: 10.1016/j.vaccine.2011.11.109.
13 Li, Y., Ma, Y., An, Z., Yue, C., Wang, Y., Wang, L., Liu, Y., Yuan, X., Zhang, S., Ye, Q., Li, H., Li, K., Yin, Z., & Wang, H. (2021). Immunogenicity of 23-Valent Pneumococcal Polysaccharide Vaccine in Patients with Chronic Obstructive Pulmonary Disease – Hebei Province, China, September-December, 2019. China CDC weekly, 3(16), 331–334. https://doi.org/10.46234/ccdcw2021.089
14 Dransfield MT, Harnden S, Burton RL, et al. Long-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2012 Sep;55(5):e35-44. DOI: 10.1093/cid/cis513.
15 Kong Yl, Zhang W, Jiang ZW, et al. lmmunogenicity and safety ofa 23-valent pneumococcalpolysaccharide vaccine inhinesehealthy population aged > 2 years: a randomized, double-blindedactive control, phase Il trial[J]. HumanVaccines andImmunotherapeutics, 2015,11(10):2425 -2433
16 Feng, J. (2018). Effectiveness and influencing factors of 23-valent pneumococcal polysaccharide vaccination among community-dwelling elderly. Electrocardiogram Journal (Electronic Edition), 7(2), 166–167.
17 Yang, Z. (2019). Safety and immunogenicity analysis of 23-valent pneumococcal polysaccharide vaccine in elderly aged 60 and above. Psychological Monthly, 14(12), 16–18.
18 Miernyk KM, Butler IC, Bulkow LR, et al. Immunogenicity andreactogenicity of pneumococcal polysaccharide and conjugatevaccines in Alaska native adults 55-70 years of age[J]. ClinicalInfectious Diseases,2009,49(2): 241 -248.
19 Serpa JA, Valayam J, Musher DM, et al. V;3 antibody response toimmunization with pneumococcal polysaccharide vaccine111middle-aged and elderly persons[J].Clinicaland VaccineImmunology,2011,18(3):362-366.
20 Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23)[J]. Morbidity and Mortality Weekly Report, 2010, 59(34):1102–1106
21 Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults[J]. Cochrane Database of Systematic Reviews, 2008(1): CD000422.
22 Gao Jie, G. J., He YongPin, H. Y., Shen Bing, S. B., Hu Hong, H. H., Bei WeiHui, B. W., & Xu ChuiWei, X. C. (2015). Evaluation of 23-valent pneumococcal polysaccharide vaccine immunization among people aged> 60 years in Jing’an, Shanghai.
23 Jia-ping YANG, Xing-tang YANG, Xiao-jun LI, Mai-yue ZHANG, Li SHEN. Effect observation on senile inoculation of 23-valent pneumococcal polysaccharide vaccine in Baoshan District of Shanghai City[J]. Shanghai Journal of Preventive Medicine, 2017, 29(12): 945-948. DOI: 10.19428/j.cnki.sjpm.20180122.005
24 Dong, B. R., Xu, Y., Zhang, Y. L., & Yue, J. R. (2005). Efficacy and safety of 23-valent pneumococcal polysaccharide vaccine in prevention of lower respiratory tract infections in the Chinese elderly: A prospective concurrent controlled trial. Chest, 128(4). https://doi.org/10.1378/chest.128.4_meetingabstracts.148s
25 Grabenstein, J. D., & Manoff, S. B. (2012). Pneumococcal polysaccharide 23-valent vaccine: Long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine, 30(30), 4435–4444. https://doi.org/10.1016/j.vaccine.2012.04.052
26 Guo X, Li J, Qiu J, Zhang R, Ren J, Huang Z, Li Z, Liang X, Lan F, Chen J, Huang F, Sun X. Persistence of antibody to 23-valent pneumococcal polysaccharide vaccine: a 5-year prospective follow-up cohort study. Expert Rev Vaccines. 2024 Jan-Dec;23(1):237-245. doi: 10.1080/14760584.2023.2296934. Epub 2024 Feb 19. PMID: 38369970.
27 LI Xiao-jun, ZHAO Shi-ying, YANG Jia-ping, ZHU Jiang, ZHANG Mai-yue, ZHANG Yue-juan. PPV-23 vaccination evaluation of the elderly in a district of Shanghai[J]. Shanghai Journal of Preventive Medicine, 2019, 31(4): 259-263. DOI: 10.19428/j.cnki.sjpm.2019.19319
28 Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine[J].The New England Journal of Medicine, 1991, 325(21): 1453 – 1460
29 Jiang, Y., Pang, H., Zhang, Z., et al. (2016). Evaluation of immune response after pneumococcal vaccination among elderly community residents in Changning District, Shanghai. Occupational and Health, 32(20), 2819–2822.
30 Qiu, J., Li, Z., Huang, F., Huang, Z., Liang, X., Li, J., Liao, Y., Guo, X., & Sun, X. (2025). Immunogenicity and safety study of 23-valent pneumococcal polysaccharide vaccine revaccination among elderly individuals aged 60–70 years in Shanghai, China. Frontiers in Immunology, 16. https://doi.org/10.3389/fimmu.2025.1623611
31 Guo, X., Qiu, J., Ren, J., Liu, J. C., & Sun, X. D. (2020). Safety evaluation of mass inoculation of 23 valent pneumococcal polysaccharide vaccine among elderly people aged 60 and above in Shanghai from 2013 to 2017. Zhonghua yu Fang yi xue za zhi [Chinese Journal of Preventive Medicine], 54(9), 929-933.
32 Chen, J., XU, J., Zhang, C., Tan, H., & LI, Z. (2016). Immunization coverage and safety of the 23-valent pneumococcal polysaccharide vaccine in Guang-zhou from 2010 to 2015. Chinese Journal of Microbiology and Immunology, 380-383.
33 WANG, Z., SUN, X., ZHANG, M., TANG, F., MA, F., XU, Y., … & LUO, L. (2019). Immunogenicity and safety of co-immunization with 23-valent pneumococcal polysaccharide vaccine and influenza virus split vaccine for children aged 3-7 years. Chinese Journal of Microbiology and Immunology, 758-762.
34 Wang, M. C., Wang, T. P., & Yang, S. M. (2010). Effectiveness of 23-valent penumococcal polysaccharide and split-virus influenza vaccines to prevent respiratory diseases for elderly people. Zhongguo yi Miao he Mian yi, 16(3), 229-232.
35 He, B., Xiang, Z., Shen, G., Du, Z., & Xu, R. (2018). Safety evaluation of influenza vaccine and 23-valent pneumococcal polysaccharide vaccine administered alone or concurrently in the elderly. Chinese Journal of Microbiology and Immunology, 38(4), 293–299.





