Currently, the available high-valent combination vaccines in the Chinese market are quadrivalent and pentavalent. Each of the above vaccines has only one manufacturer to produce them. The DTaP-Hib quadrivalent vaccine is produced by Beijing Minhai Biotechnology Co., Ltd., a wholly owned subsidiary of Shenzhen Kangtai. Sanofi Pasteur SA supplies the DTaP-IPV-Hib pentavalent vaccine.
Combination vaccines launched in China
| Quadrivalent Vaccine | Pentavalent Vaccine | |
| Vaccine abbreviation | DTaP-Hib | DTaP-IPV-Hib |
| Manufacturer | Beijing Minhai Biotechnology Co., Ltd. (Subsidiary of Shenzhen Kangtai) | Sanofi Pasteur SA |
| Vaccination schedule | At 3/4/5/18 months | At 2/3/4/18 months |
| A total of 4 doses | A total of 4 doses | |
| Age of vaccination | >3 months old | >2 months |
| Dose unit | 0.5ml | 0.5ml |
Data source: NMPA, official websites of Minhai Biotechnology and Sanofi
Pediatric Quadrivalent and Pentavalent Vaccine Lot Release Data
Due to the impact of the large-scale administration of COVID-19 vaccines, the overall vaccine lot release in China decreased since 2021. The quadrivalent vaccine’s lot release decreased by 18% from 2020 to 2021 and 26% from 2021 to 2022. However, a recovery trend was observed since 2022, with an increase of 78% in the fourth quarter of 2022 (16 lots) vs. Q4 of 2021 (9 lots).
The pentavalent vaccine experienced a 15% decrease in lot release from 2020 to 2021, followed by a 27% increase from 2021 to 2022. In 2022 Q4, the pentavalent vaccine has 12 lots released, with a decline of 43% compared with Q4 of 2021 (21 lots).
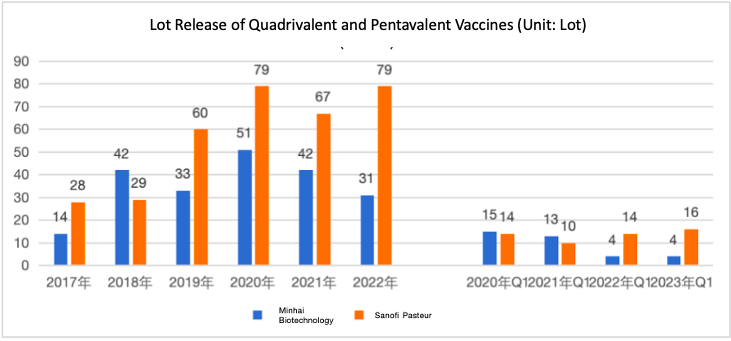
In 2022, the quadrivalent vaccine of Beijing Minhai Biotechnology had 310k doses released annually (-26% vs. 2021). The pentavalent vaccine of Sanofi Pasteur had 790k doses released (+17.91% vs. 2021). In Q1 2023, Minhai Biotechnology had 440k doese released for its quadrivalent vaccine (0% vs. Q1 2022), while Sanofi has 160k doses increased for the pentavalent vaccine (in total 1280k doses, +14.29%) compared with Q1 2022.
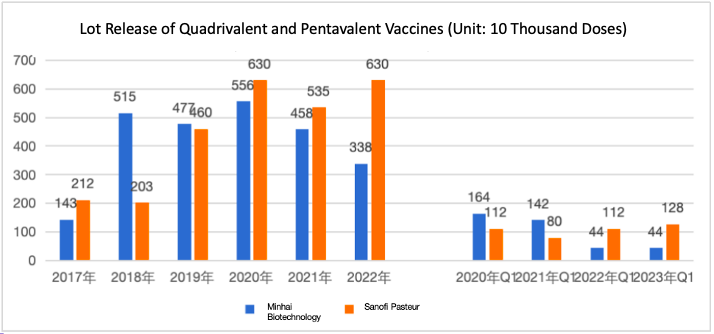
From 2020 to 2022, the annual lot release of domestically produced quadrivalent vaccines decreased, with 5.56 million doses in 2020, 4.58 million in 2021, and 3.38 million in 2022. Over the same period, the imported pentavalent vaccine’s lot release fluctuated, with 6.3 million doses in 2020, 5.35 million in 2021, and returned to 6.3 million in 2022.
NMPA Rules for Vaccine Manufacturing and Distribution
To strengthen the implementation of the Vaccine Administration Law to regulate the manufacturing and distribution of vaccines, the National Medical Products Administration (NMPA) initiated two rounds of public consultations on the “Rules for Vaccine Manufacturing and Distribution” in 2020 and 2021, which came into effect on July 8, 2022.
Before the effectiveness of the Rules, early regulations mandated that only pharmaceutical companies could possess a Drug Approval License. Manufacturers who plan to produce combination vaccines must first hold the licenses of the respective monovalent vaccines. Different manufacturers (even manufacturers under the same parent company) cannot jointly develop combination vaccines.1
Following the release of the Rules, a Marketing Authorization Holder (MAH) system was established across the spectrum of vaccine development, registration, manufacturing, and distribution, similar to what has been implemented for common drugs. The Rules list the conditions for MAH to apply for commissioned manufacturing, the requirements for both the commissioning and commissioned parties, and the specific processes involved in commissioned production, which enables the joint R&D of combination vaccines. 2
Content Editor: Menglu Jiang
Page Editor: Ziqi Liu
Reference
- Zhao Yangsheng et al. Development status, problems and countermeasures of the joint vaccine industry in China. Chinese Pharmaceutical Industry Journal.2018,49(9). https://xueshu.baidu.com/usercenter/paper/show?paperid=1g2k0cv0kc1002n0we0k0mt0h4658951&site=xueshu_se. Accessed Sep. 22, 2024. (In Chinese)
- National Drug Administration. Regulations on the Administration of Vaccine Production and Circulation. https://www.nmpa.gov.cn/xxgk/fgwj/xzhgfxwj/20220708185734126.html. Accessed Sep. 22, 2024.





